Ensuring that food products meet the microbial standards set for each type of food is, of course, essential. However, in the case of voluntary testing, the effectiveness of microbial testing of products at the time of shipment varies. Some foods benefit significantly from such tests, while for others, the efficacy is minimal. So, which foods benefit from microbial testing at shipment, and which do not? This article introduces a scientific paper that investigates this issue. The author is Dr. Zwietering, Chair of the International Commission on Microbiological Specifications for Foods (ICMSF) as of May 2022.
International Commission on Microbiological Specifications for Foods (ICMSF): An academic organisation established in 1962 to collect and provide scientific information on microbiological standards for foods. The ICMSF advises organisations such as the Codex Alimentarius Commission and the FAO/WHO on issues like the establishment of sampling consistency in food microbiological testing.
Zwietering et al.:
Relevance of microbial finished product testing in food safety management
Food Control, 60, 31-43(2016)
Open access
This review scientifically examines the validity of microbial tests related to both the food products at the time of shipment and the production environment of the factory, using case studies of three types of food: canned goods (considering the hazard of Clostridium botulinum), chocolate (considering the hazard of Salmonella), and cooked sliced ham (considering the hazard of Listeria monocytogenes). These three types of food have different characteristics in terms of sterilisation processes, the potential for subsequent contamination, and the possibility of microbial growth within the food.

Canned Products
Manufacturing Process and Product Characteristics
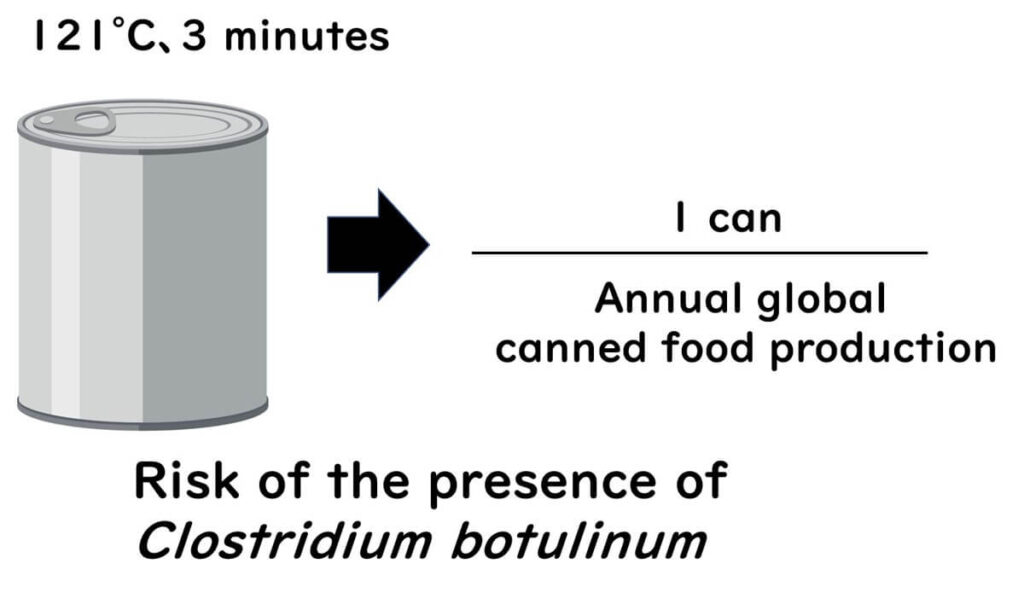
In sealed canned foods, the sterilisation process targets the spores of Clostridium botulinum, commonly using an F121°C value of 3 minutes. Considering that the D121°C value for type I botulinum is 0.21 minutes, this results in a 14.3 log reduction. If 100 spores are present in a can, this means that only 1 in 10¹² spores would survive, equivalent to the annual global production of canned goods.
Assuming different initial levels would yield varying defect rates, but with a treatment of over 12D, there would practically be no botulinum spores remaining.
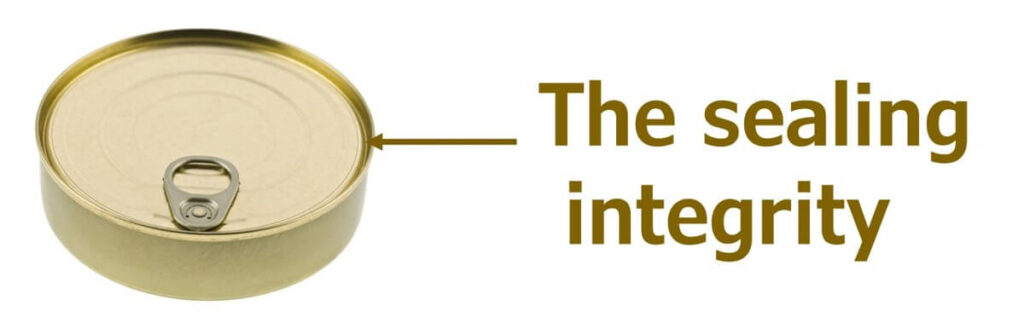
If the can remains completely sealed, there is no chance of recontamination, and the microbial count will solely depend on the level of surviving microbes. In cases where sealing is not perfect, contamination with microbes other than Clostridium botulinum could occur. However, given that canned foods are stored in aerobic environments, there is potential for spoilage bacteria, but almost no chance for botulinum recontamination.
Is Microbial Testing of Food at Shipment Effective?
For canned products, even a single contaminated can in 100,000 is unacceptable, making end-product testing impractical. If one can in 100,000 is contaminated, this cannot be tolerated. To guarantee with 95% certainty that no cans contain surviving spores, 95% of the cans would need to be sampled—a clearly unrealistic scenario. Even if all 95% tested negative, there’s no assurance that the remaining 5% are free of spores. Just a few cans in the untested 5% containing botulinum spores could cause a catastrophe.
In summary:
End-product microbial testing is entirely ineffective.
The only viable option for food safety management is the CCP management of HACCP.

Chocolate Products
Manufacturing Process and Product Characteristics
Chocolate is generally considered microbiologically safe. However, since it is often consumed directly and is popular among vulnerable groups like children, even low levels of Salmonella can pose an infection risk. In 2020, a global Salmonella outbreak linked to Belgian chocolate underscored this danger.
- Salmonella can be present in raw or fermented cocoa beans, which may not always be processed or stored under the most hygienic conditions in their countries of origin. However, processes like roasting and steaming during cocoa processing are likely to eliminate Salmonella from raw beans. Thus, if the processing into liquid chocolate is well-managed, the presence of Salmonella in liquid chocolate should be minimal.

- Subsequent steps in the production of chocolate products can introduce secondary contamination from the environment or from additives like milk powder, nuts, and dried fruits.

- Chocolate’s low water activity prevents microbial growth during storage, but its high fat content and low water activity can give Salmonella heat resistance and allow it to survive for long periods. Fat can also protect microbes from stomach acid during digestion. Outbreaks linked to chocolate products often involve low levels of Salmonella, such as 4.3 cfu/100 g or 1 cfu/25 g.

Is Microbial Testing of Food at Shipment Effective?
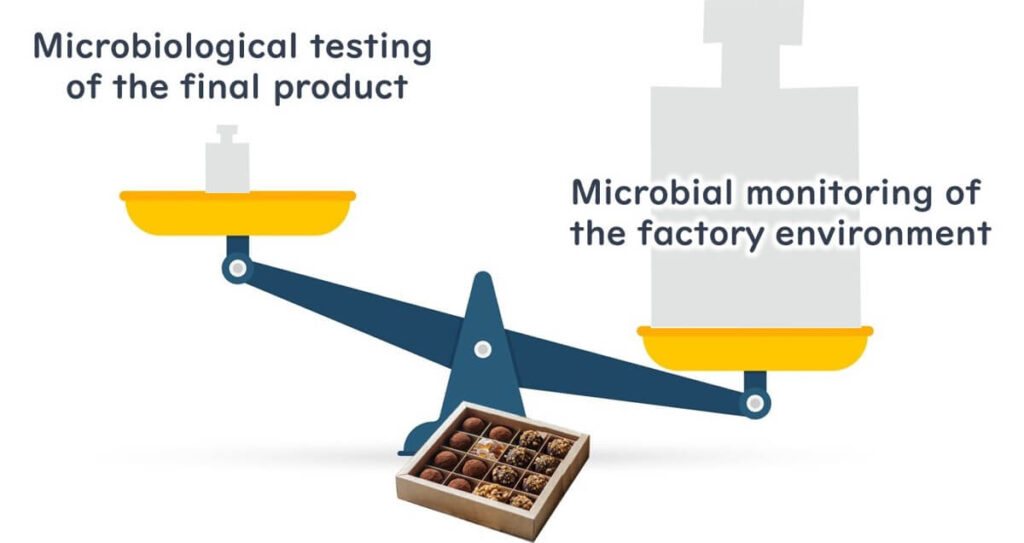
Given the low prevalence of Salmonella in chocolate, end-product testing alone cannot reliably detect contamination. Relying solely on such testing can create a false sense of security.
It is crucial to manage secondary contamination. Environmental sampling of the production environment and equipment is effective for this purpose. While detecting Salmonella is challenging due to its low presence, testing for indicator bacteria like Enterobacteriaceae is recommended for a more reliable assessment.
Ham Products
Manufacturing Process and Product Characteristics
For boneless ham, as examined in this paper, the manufacturing process involves cutting the pork muscle to remove the bone, curing, tumbling, forming in plastic casings, and finally, heat treatment.
During heat treatment, the core temperature exceeds 70°C with a pasteurisation target of over 40 minutes (P70°C > 40). This process is sufficient to inactivate Listeria monocytogenes cells that may have been introduced with the raw material or during pre-cooking processes.
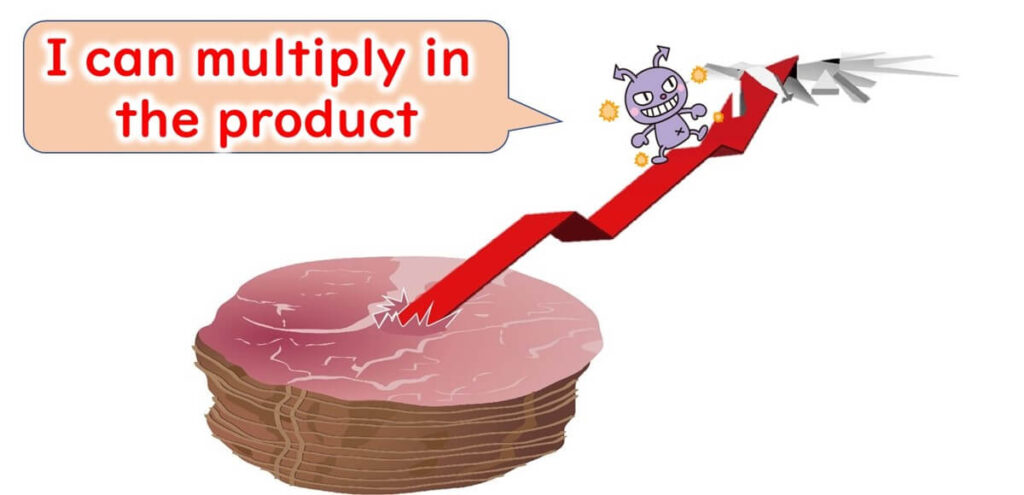
However, the potential for recontamination with Listeria exists during the mechanical slicing of the ham. Among the three products studied, cooked ham is considered to have the highest risk of recontamination. Therefore, it cannot be assumed that the Listeria contamination rate in the shipped product is low. Cross-contamination between products and biofilm formation in the factory are possible sources of recontamination. Previous literature estimates that, on average, 3.2% of sliced ham products available in retail are contaminated with Listeria monocytogenes..

Though sliced ham is stored refrigerated until consumption, Listeria can proliferate on the ham.
Is Microbial Testing of Food at Shipment Effective?
- Pre-sale end-product testing holds some significance.
Since cooked ham is most susceptible to end-point contamination and Listeria can proliferate in the finished product, end-point sampling is deemed effective. However, assuming a contamination rate of 3.2%, a sampling plan would require 92 samples (c=0, n=92, 25g/sample) to guarantee 95% certainty in detecting defective ham products. Testing 92 samples is far more extensive than standard sampling plans for Listeria in deli products. For example, the European Commission (2005) recommends sampling five samples for ready-to-eat refrigerated foods where Listeria proliferation is possible. Dr. Zwietering suggests interpreting the results of testing five final product samples as follows:
- It does not prove a contamination rate below 3.2% for a specific batch.
- It is meaningful in detecting significant deviations.
- It provides evidence that CCPs are functioning correctly.
In conclusion, the contamination rate (both rate and count) of cooked ham is too low to be efficiently detected by end-product microbial testing. Furthermore, raw material testing is unnecessary as the cooking process inactivates Listeria, assuming the HACCP plan is implemented.
The most appropriate management measure is preventing Listeria recontamination during slicing. This can be achieved through proper management of cleaning and operations around slicers and packaging equipment.

Summary
For canned products, processing occurs in a sealed state, with high-temperature inactivation yielding a significant reduction in microbial count and minimal risk of recontamination. Post-inactivation, canned products are assumed free of Clostridium botulinum spores, eliminating the need for microbial testing at shipment.
In chocolate, microbial inactivation during processing is effective, but recontamination can occur in subsequent stages. Due to low water activity, microbial growth is unlikely. Nonetheless, contamination is rare, making microbial testing at shipment unnecessary.
In cooked sliced ham, microbial inactivation during cooking is highly effective, but recontamination and microbial growth in the product can occur. Therefore, at least for verification purposes, microbial testing at shipment is recommended, with caution in interpreting results as outlined.
My Take on This Study

From my perspective, the key takeaway is how clearly this paper challenges the assumption that microbial testing at shipment is always a meaningful food safety control. What struck me most in this study is the stark contrast between food types: while microbial testing is virtually meaningless for canned goods due to their sterility, it can play a valuable role—albeit limited—for products like sliced ham that are prone to post-process contamination.
This study reinforces a point I often stress in food safety training: microbial testing should never be treated as a standalone measure. It must be understood in the context of processing steps, the potential for recontamination, and microbial growth characteristics. For quality control professionals, the implication is clear—rely on testing as a verification tool, not as your primary defence.
This confirms what I’ve long emphasised: effective HACCP implementation and environmental monitoring are far more impactful than overreliance on finished product testing, especially for low-prevalence hazards.
For related articles, check out our beginner-friendly summary on the role and position of microbial testing in HACCP. It’s a great resource for understanding how microbial testing fits into the larger framework of food safety management:
We also have another article explaining the calculation methods for the accuracy of microbial testing in food, tailored for beginners. Don’t miss it: