Real-Time PCR, particularly the TaqMan method, is transforming microbiological testing in the food industry. Its ability to provide precise, rapid DNA quantification makes it an invaluable tool for quality control professionals. This article explains the TaqMan method, its key advantages for on-site applications, and how it simplifies the detection of target DNA in food safety management.
What is Real-Time PCR?
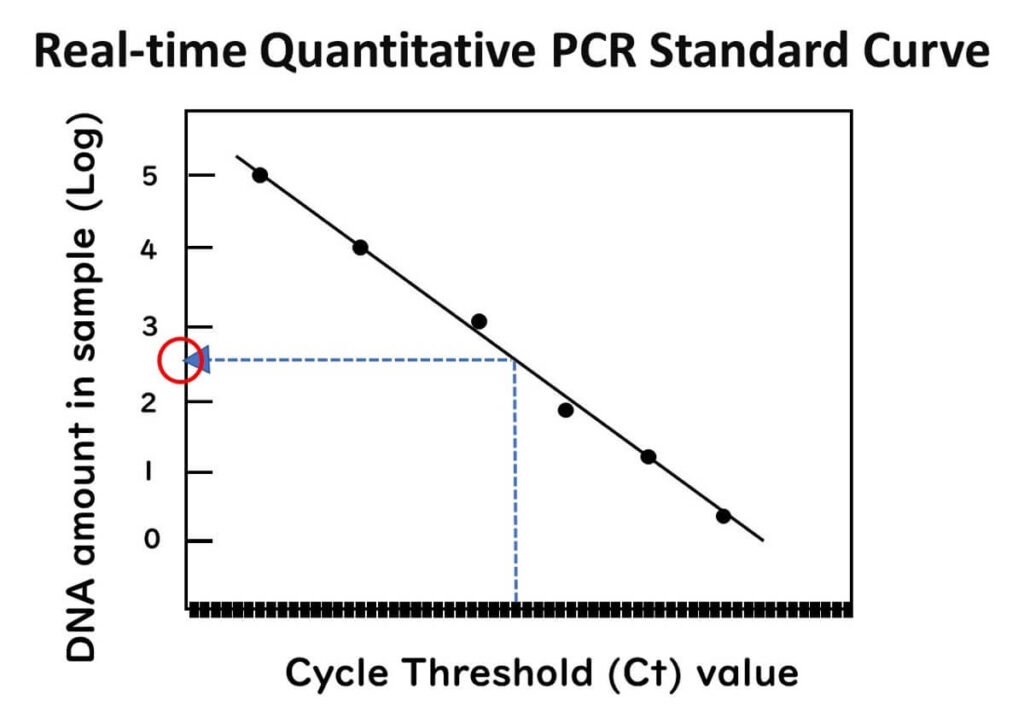
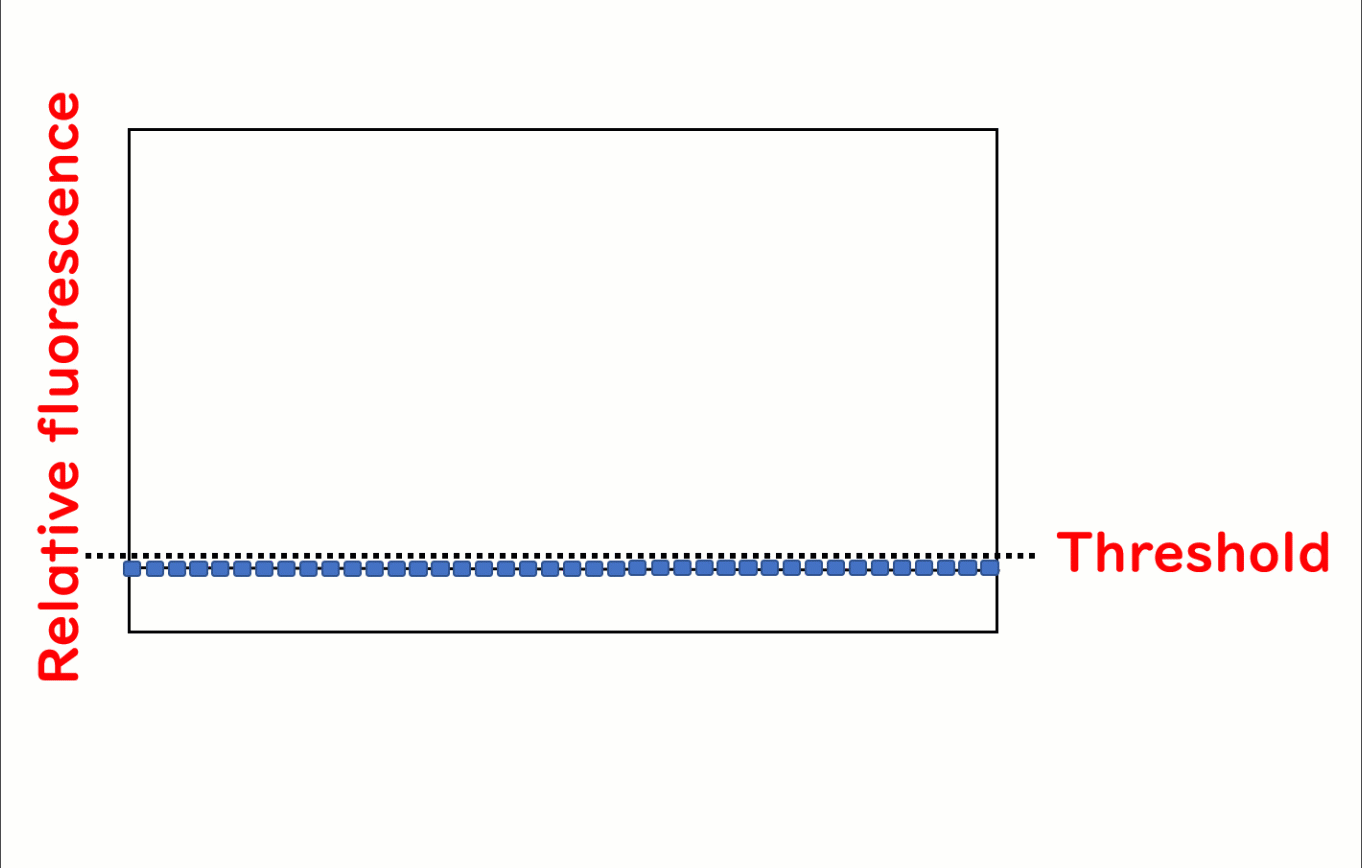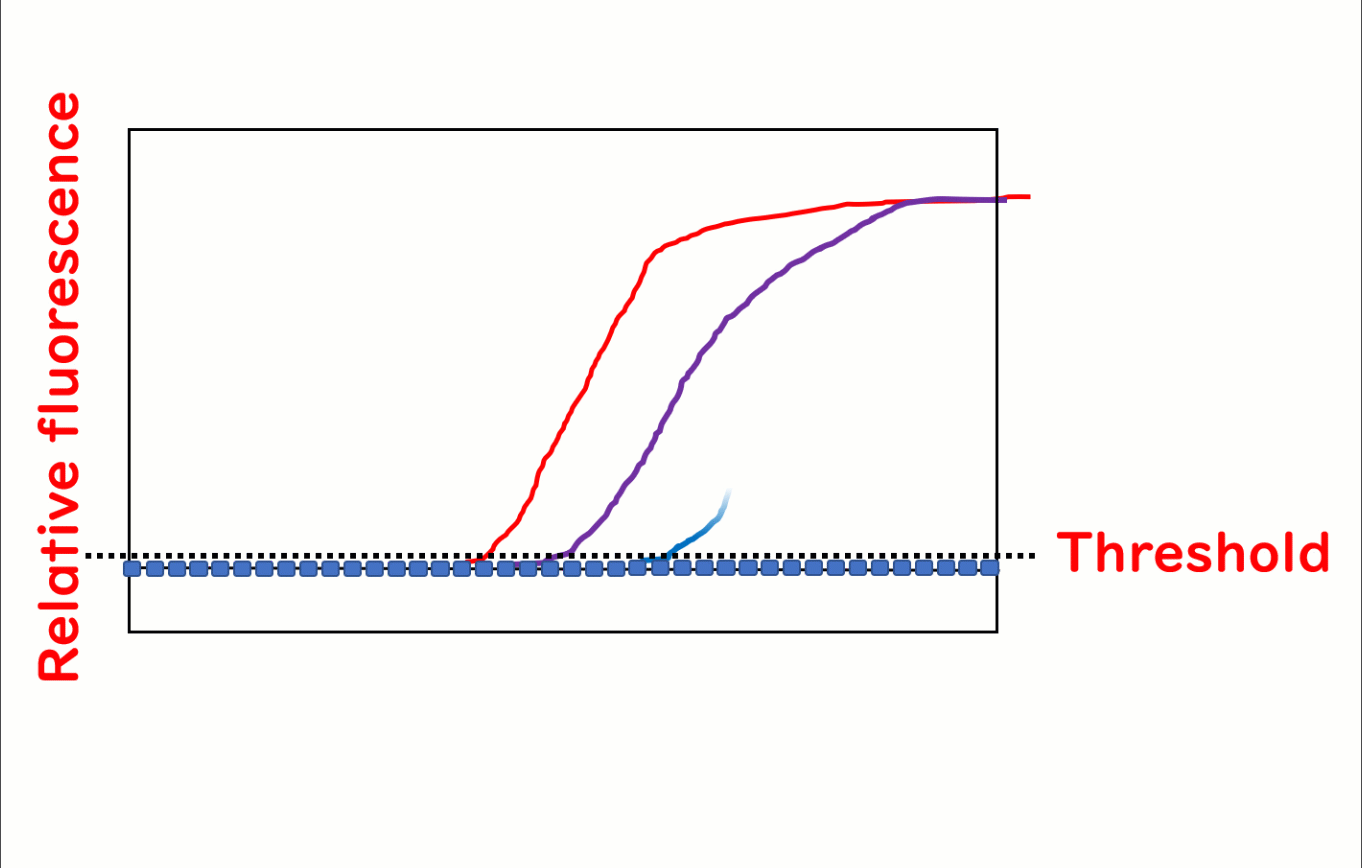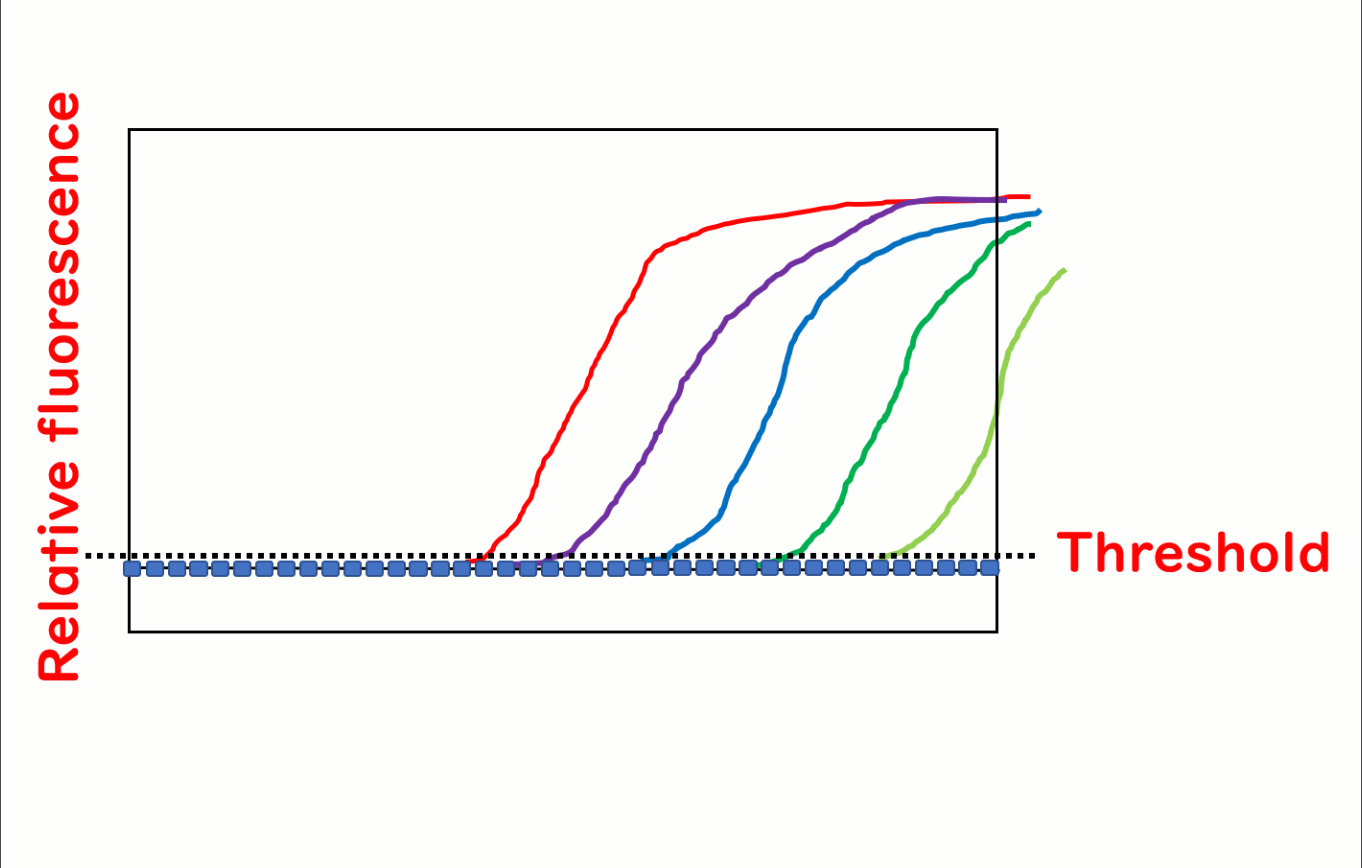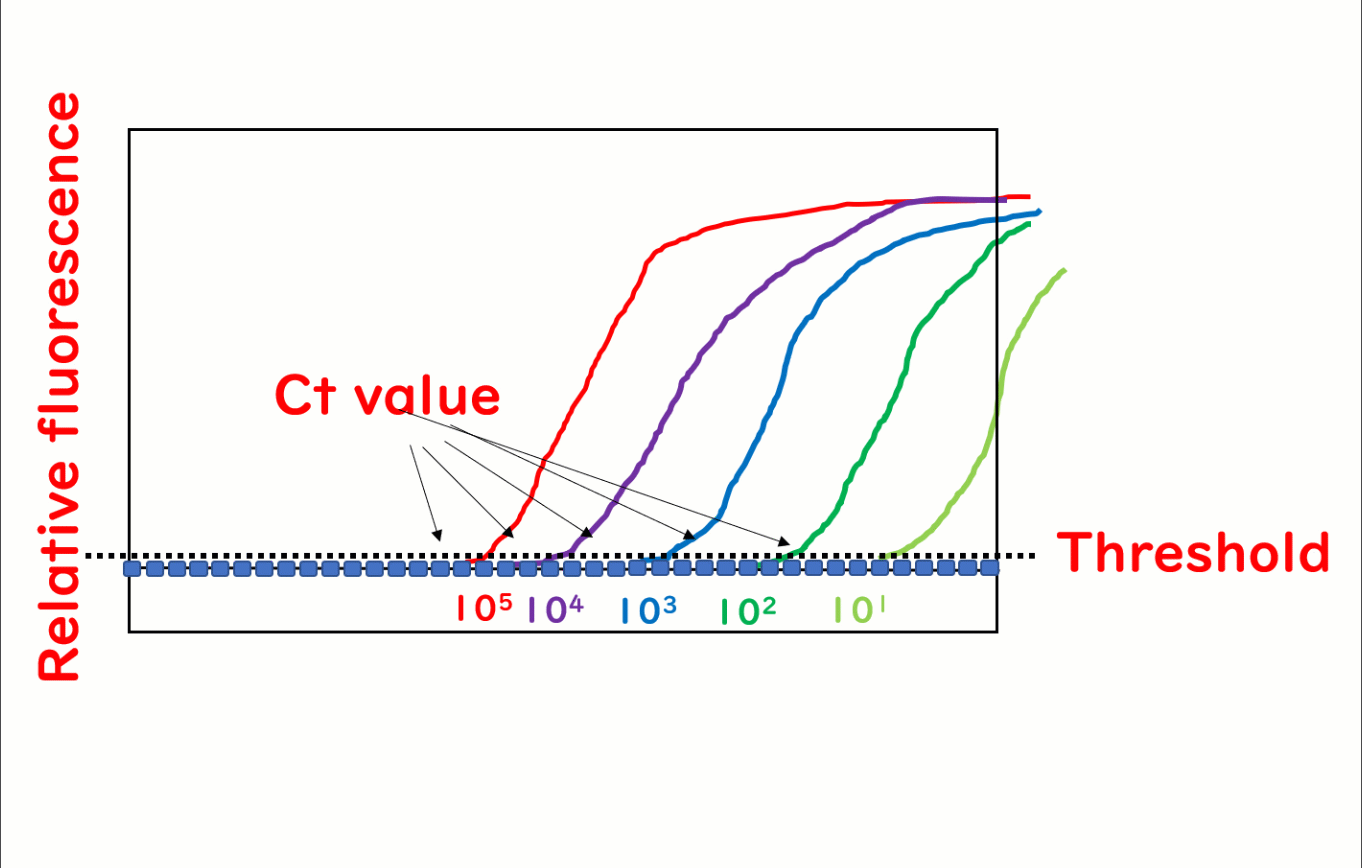
Real-time PCR, also known as quantitative PCR (qPCR), is a cutting-edge technique widely used in food safety testing to quantify DNA. During the PCR process, fluorescence is emitted as the reaction progresses, and its intensity increases with each cycle. The number of PCR cycles required for the fluorescence to exceed a predetermined threshold—referred to as the cycle threshold (Ct)—is directly proportional to the initial quantity of target DNA in the sample. This makes it possible to precisely measure DNA concentrations. For simplicity, this article refers to the method as real-time PCR.
The TaqMan Method
Various fluorescent probes have been developed for real-time PCR, with the TaqMan probe method being one of the earliest and most widely adopted. Understanding this method not only provides insight into the TaqMan approach but also lays the foundation for grasping other fluorescence-based real-time PCR techniques.
The TaqMan method differs from standard PCR by incorporating a third fluorescent probe, in addition to the two primers flanking the target gene. This probe is specifically designed to anneal within the target DNA region, making it a crucial component for detecting and quantifying DNA during amplification.
The TaqMan Probe
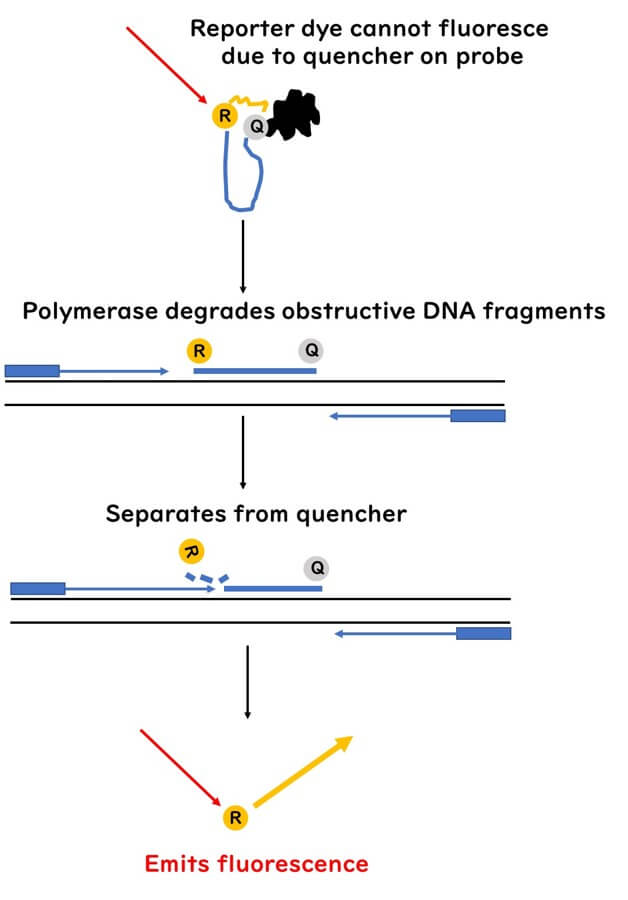
The TaqMan probe features a unique design with two dyes attached to its ends. At the 5' end is the reporter dye, which emits fluorescence when excited by light. At the 3' end is the quencher dye, which absorbs the energy emitted by the reporter dye, suppressing fluorescence while the probe remains intact.
When the probe is bound to the target DNA, the quencher dye's proximity to the reporter dye ensures that no fluorescence is emitted. This quenching mechanism is key to distinguishing between bound and cleaved probes during the PCR process.
How It Works
During PCR, DNA polymerase extends a new DNA strand from the primers. When the polymerase encounters the probe, its 5'-3' exonuclease activity cleaves the probe, separating the reporter dye from the quencher dye. This separation allows the reporter dye to emit fluorescence.
As the amplification cycles progress, more probes are cleaved, resulting in an increase in fluorescence intensity. The fluorescence signal directly correlates with the presence and quantity of the target DNA in the sample, making this method both highly sensitive and specific.
Measuring Fluorescence
The emitted fluorescence is measured using a 96-well plate fluorometer, a device capable of simultaneously analyzing multiple samples. By tracking the fluorescence intensity, the presence or absence of the target DNA can be determined. This approach eliminates the need for traditional gel electrophoresis, streamlining the detection process.
Summary
Real-time PCR, particularly the TaqMan method, is an invaluable tool for food safety testing and other DNA quantification applications. By leveraging fluorescent probes, it offers real-time insights into the amplification process, providing high accuracy and efficiency in detecting and quantifying target DNA. Its speed, simplicity, and quantification capabilities make it an essential technique for on-site applications in the food industry.
Key Points of Real-Time PCR Method
The advantages of real-time PCR, particularly for food safety applications, can be summarised in the following three points:
(1)Simplicity
Advanced technologies must be practical and accessible for on-site use. Real-time PCR simplifies the process, allowing personnel without a microbiology background to achieve reliable results after minimal training (e.g., one week).
Traditional PCR methods require labor-intensive steps, such as gel preparation and the use of carcinogenic ethidium bromide for detection, making them less ideal for routine food testing. In contrast, real-time PCR eliminates these complexities. After amplification, simply place the reaction tubes in a fluorometer, press the analysis button, and retrieve the results. This streamlined workflow makes it highly suitable for on-site quality control tasks in food companies.

(2)Speed
Real-time PCR significantly reduces processing time by eliminating the need for gel electrophoresis. For instance, using a 96-well plate, up to 96 samples can be analyzed within 15 minutes.
This rapid turnaround is critical in the food industry, where quick decision-making is essential. The ability to perform large-scale screening in compact setups (e.g., palm-sized 96-well plates) optimizes testing efficiency. As noted by the author, once accustomed to the TaqMan method for high-throughput analysis, reverting to traditional gel-based PCR methods feels unnecessarily tedious.
(3)Quantification
A major advantage of real-time PCR over traditional methods is its ability to quantify DNA. The fluorescence threshold (Ct value) corresponds inversely to the initial DNA concentration in the sample.
By plotting the Ct value against known DNA quantities, calibration curves can be generated, enabling precise quantification. This capability is particularly valuable for food safety testing, where accurate measurements are often required to ensure regulatory compliance and product quality.