Discover the science and applications of acidic electrolyzed water (hypochlorous acid water), a powerful antimicrobial agent overcoming the pH sensitivity of sodium hypochlorite. In this article, we explore its production process through electrolysis, its superior germ-killing properties, and its widespread adoption in the food industry. Learn how this innovative solution is shaping global food safety practices, with specific insights into its approval and usage in Japan.
What Is Acidic Electrolyzed Water?
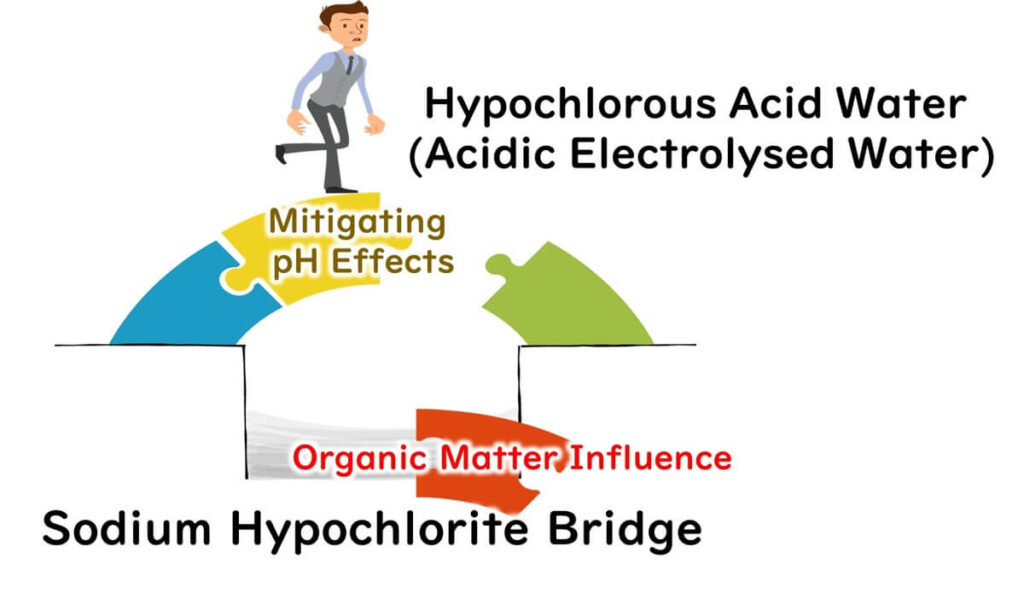
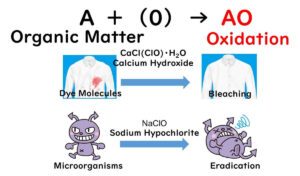
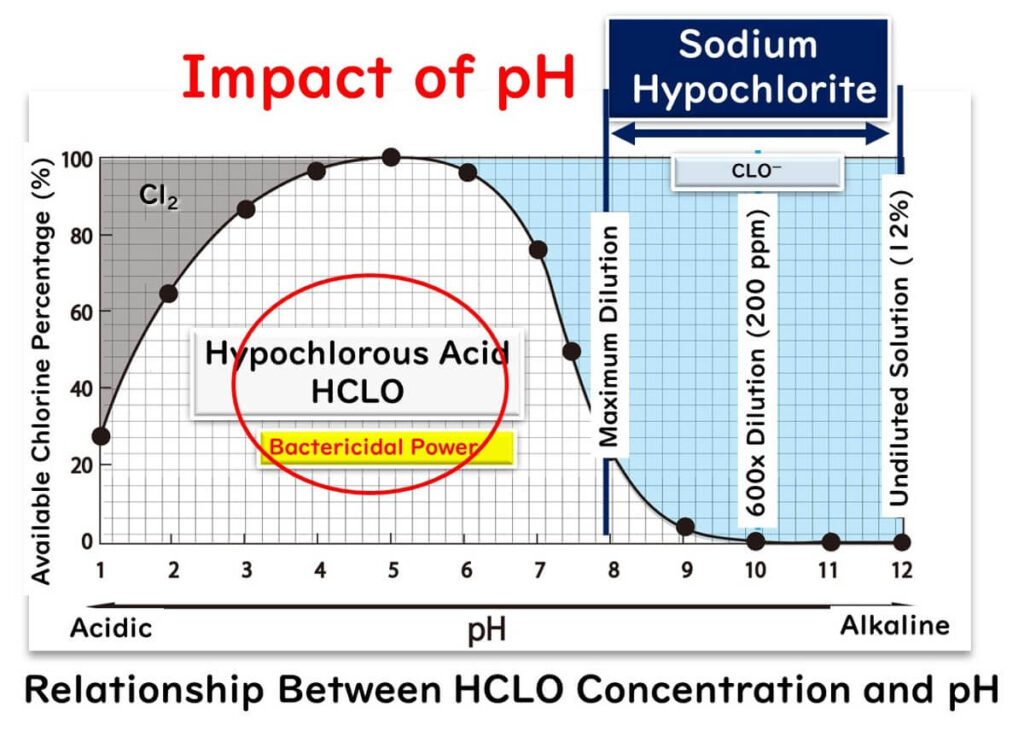
Acidic electrolyzed water, also known as hypochlorous acid water, is an advanced disinfectant that excels in overcoming one of the key limitations of sodium hypochlorite: its dependence on pH levels for effectiveness. Unlike sodium hypochlorite, hypochlorous acid operates in a lower pH range, where its composition is predominantly hypochlorous acid (HCLO). This makes it significantly more effective in microbial inactivation, as highlighted in our previous article on sodium hypochlorite.
How Is It Produced?
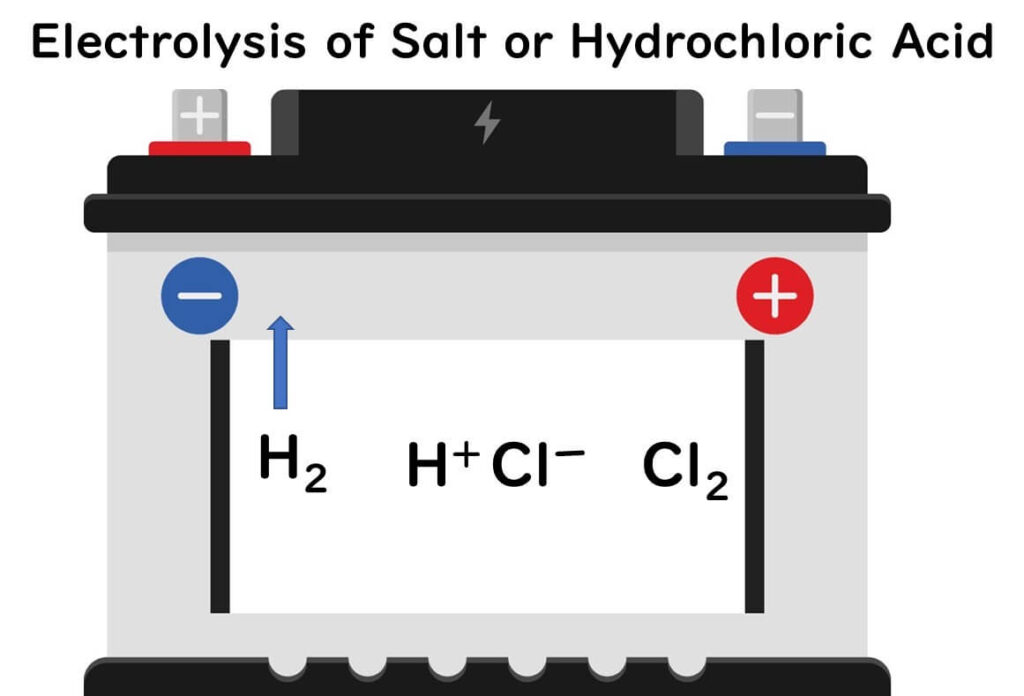
The production of acidic electrolyzed water relies on the process of electrolysis, typically involving saltwater or hydrochloric acid. Here’s a simplified breakdown of the chemical process:
- Electrolysis of Hydrochloric Acid:
- Hydrochloric acid separates into hydrogen ions (H⁺) and chloride ions (Cl⁻).
- The chloride ions migrate to the positive electrode, forming chlorine gas.
- Hydrogen ions move towards the negative electrode, releasing hydrogen gas.
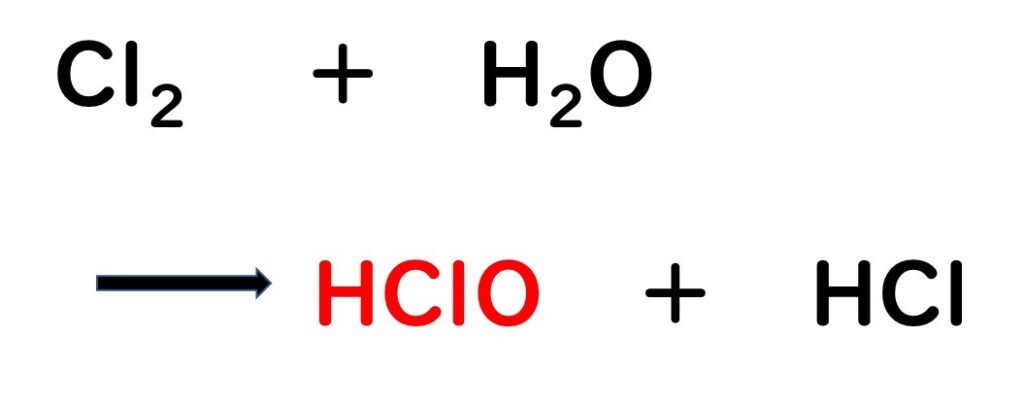
- Formation of Hypochlorous Acid:
- The chlorine gas dissolves in water, producing hypochlorous acid (HCLO), which is the active antimicrobial component of acidic electrolyzed water.
Applications in the Food Industry
Acidic electrolyzed water has become a popular disinfectant in food processing industries worldwide due to its potent germ-killing properties. Its applications, however, are regulated differently depending on the country.
- Japan’s Adoption Timeline:
- In 2012, Japan approved hypochlorous acid water for disinfecting fresh vegetables.
- In 2014, its usage was extended to fresh fish and shellfish.
These regulatory milestones highlight the increasing trust in hypochlorous acid water as a reliable tool for ensuring food safety.
Why Choose Acidic Electrolyzed Water?
Hypochlorous acid water offers a dual advantage:
- It provides enhanced microbial disinfection due to its optimal pH range.
- It mitigates the limitations of traditional chlorine-based disinfectants, particularly under varying pH conditions.
By adopting this innovative solution, food processors can achieve higher standards of hygiene while complying with strict safety regulations.