Biofilms pose a significant challenge in maintaining hygiene in food factories. These microbial communities are highly resistant to cleaning and disinfectants, often leading to unexpected contamination. This article delves into the nature of biofilms, their formation mechanisms, the physiological traits that make them resistant, the areas in food factories most prone to biofilm formation, and practical strategies for effective removal.
What Are Biofilms and Why Do They Matter?
Have you ever woken up in the morning and felt a slimy layer on your teeth? That’s a biofilm. The stubborn plaque at the base of your teeth is another common example of how biofilms develop and resist removal. Now, imagine similar layers forming in hard-to-clean areas of a food factory.
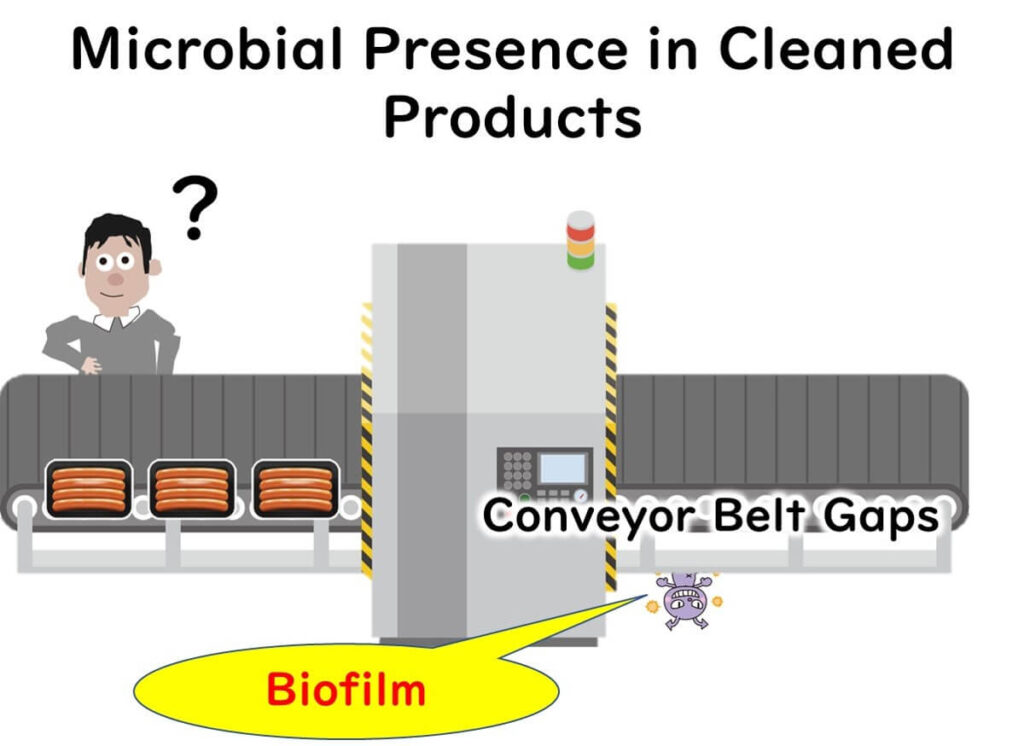
Despite regular cleaning and hygiene protocols, food manufacturing plants sometimes experience unexplained microbial contamination. The culprit? Biofilms—clusters of microorganisms that adhere to surfaces and form a protective matrix. These biofilms can persist in hidden or overlooked areas along the production line, posing a significant threat to food safety and quality.
Why Biofilms Are Highly Resistant to Disinfectants
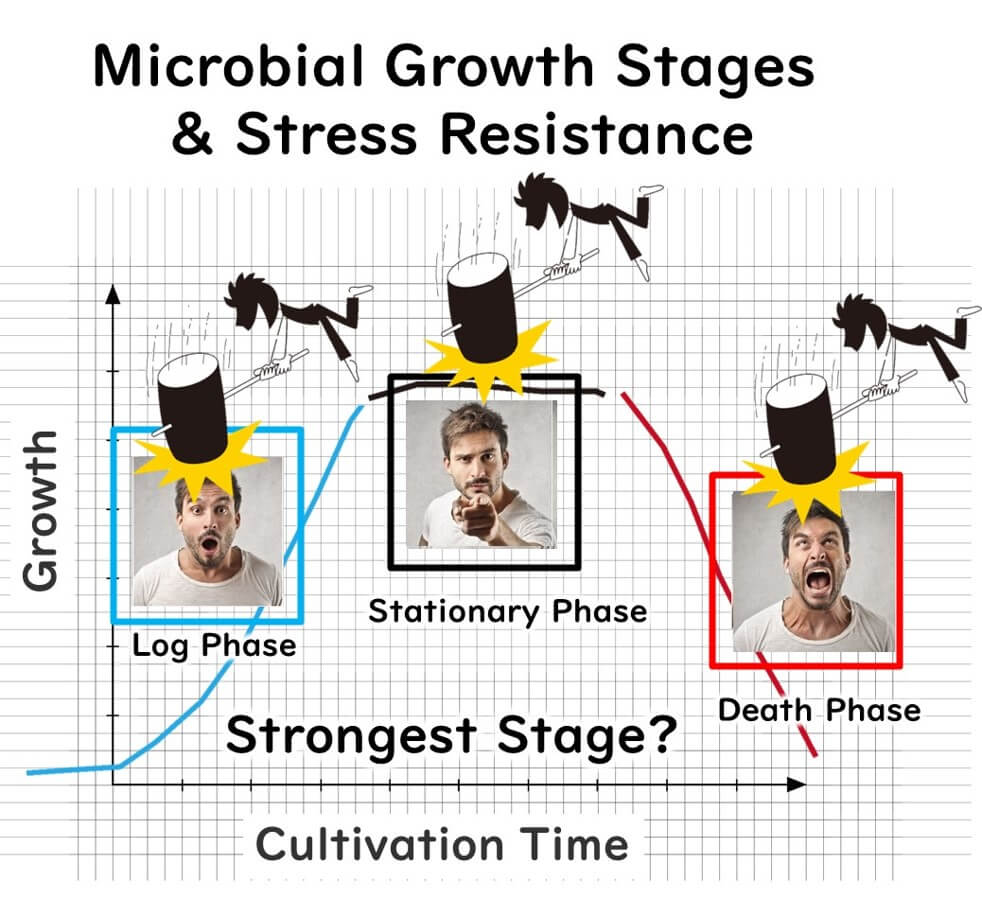
To understand why biofilms are so resilient, let's first consider the growth phases of microbes. Microbes multiply rapidly during the logarithmic growth phase, but as nutrients deplete, they enter the stationary phase, where growth slows. Eventually, they move into the death phase, where most cells gradually die.
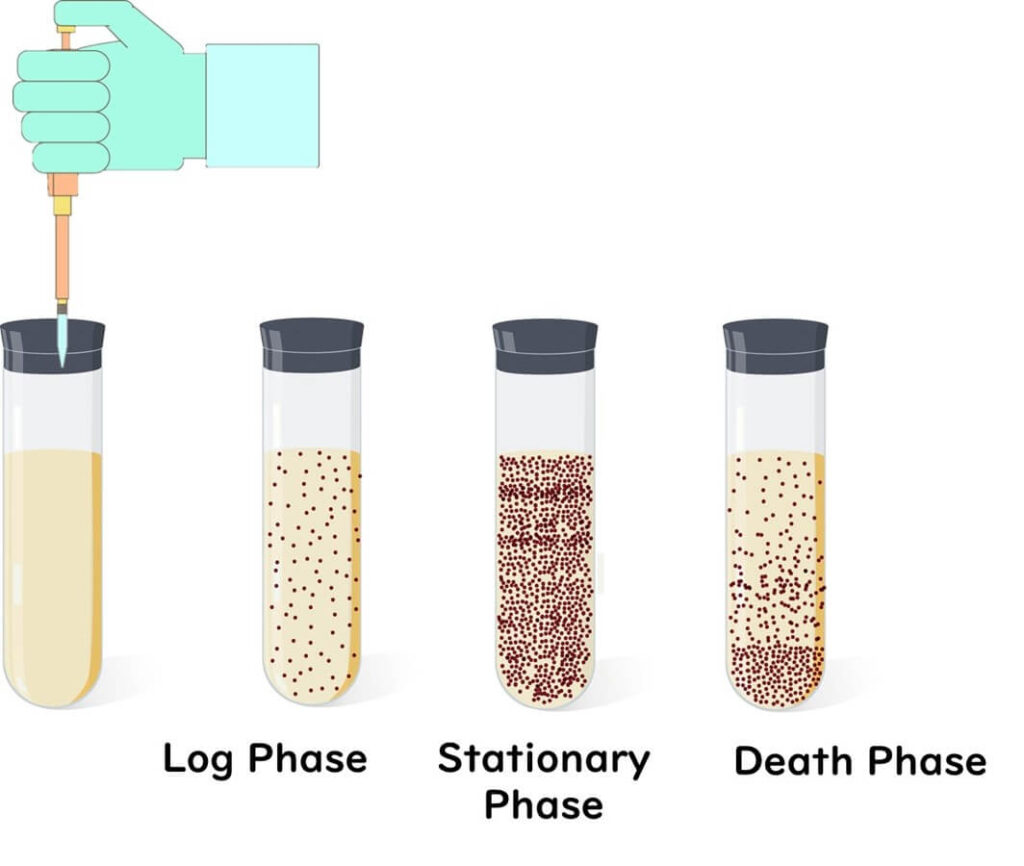
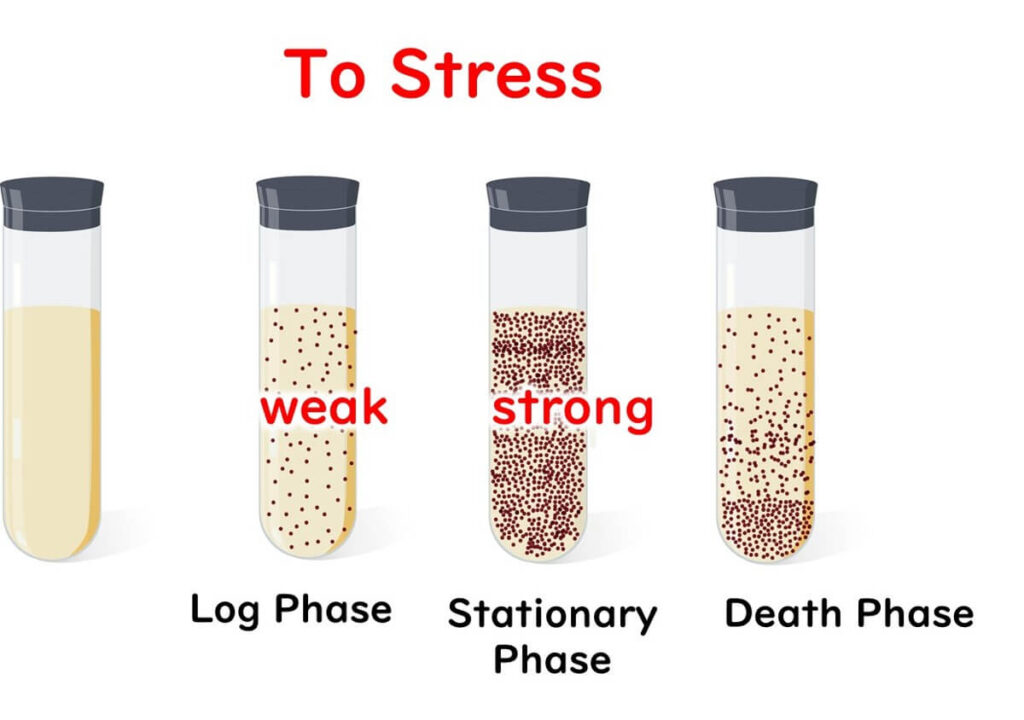
Now, if microbes were exposed to stress, which phase would make them most resilient? While many assume that rapidly multiplying cells in the logarithmic phase are strongest—much like the vitality of humans in their twenties—the truth is that microbes in the stationary phase are far more stress-resistant.
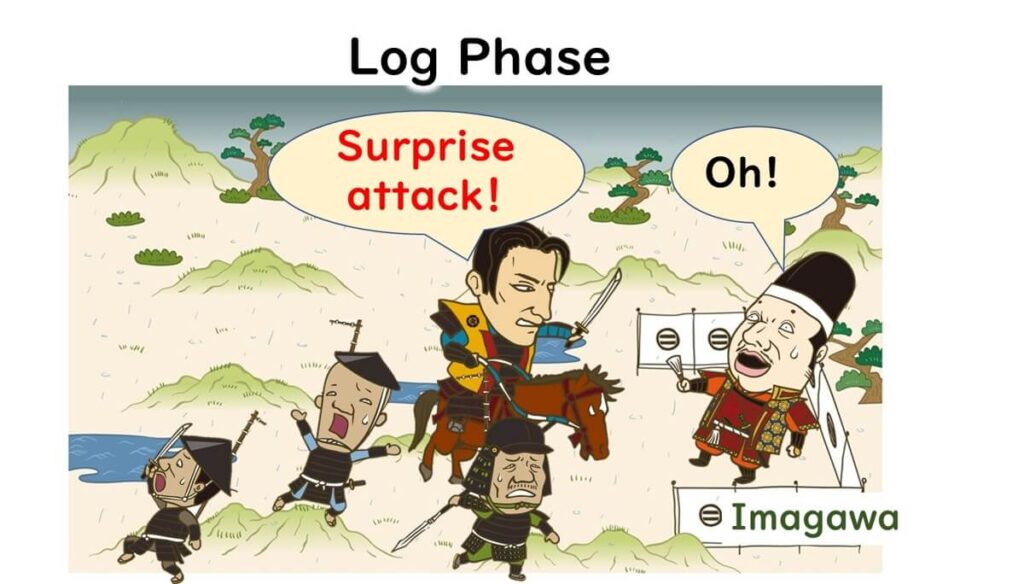
Here’s an analogy:
In the Battle of Okehazama during 16th century Japan, Oda Nobunaga, though vastly outnumbered, defeated Imagawa Yoshimoto through a surprise attack. Like overconfident armies, microbes in the logarithmic growth phase are vulnerable because they’re focused on expansion rather than defense.
In contrast, a castle under siege represents a different strategy. A fortified castle, even with fewer defenders, can resist a larger, attacking force. This principle also applies to microbes in the stationary phase or biofilm state.
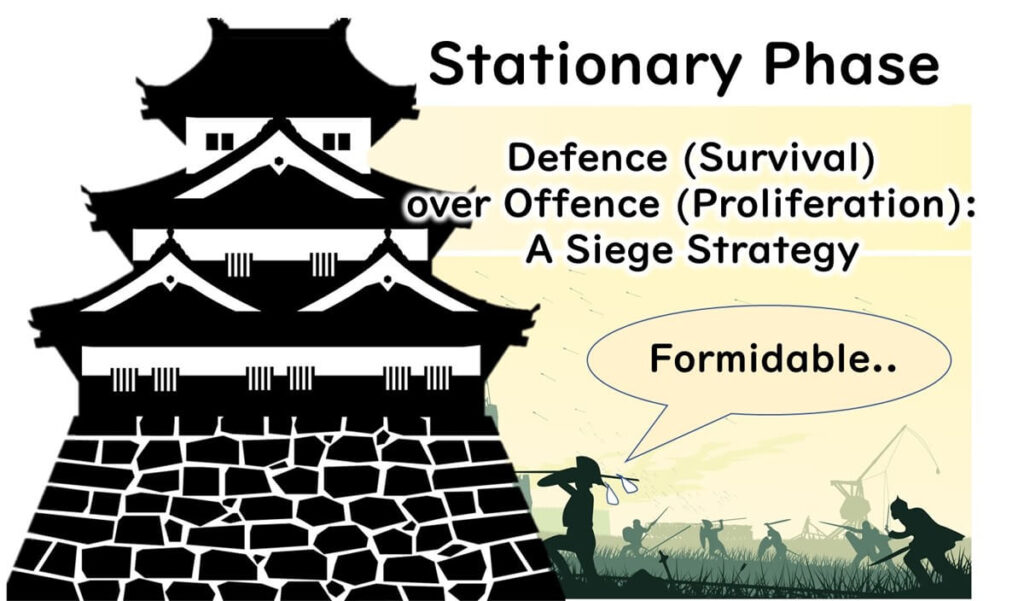
These cells, much like defenders in a castle, are highly resistant to attacks due to their strategic adaptations and preparedness.
Biofilm Resistance in Factory Settings
Microbial cells in biofilms exhibit extraordinary resistance to disinfectants for two reasons:
- Physical Barrier: Biofilms create a protective matrix of organic matter and cells that limits disinfectant penetration.
- Cellular Adaptation: Even when biofilm cells are removed from their matrix, experiments show they remain more resistant than test-tube-grown cells. This suggests a fundamental physiological change in biofilm cells, driven by stress proteins.
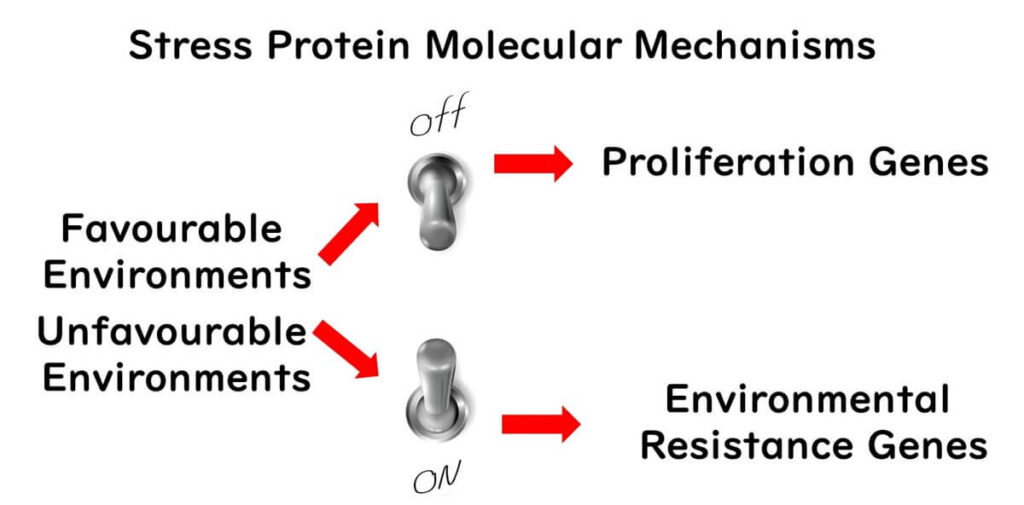
In factory environments, microbes face nutrient-scarce conditions. As a survival strategy, they attach to surfaces and form biofilms. These biofilms not only protect the microbes but also transform their cellular properties, making standard cleaning and sterilization measures less effective.
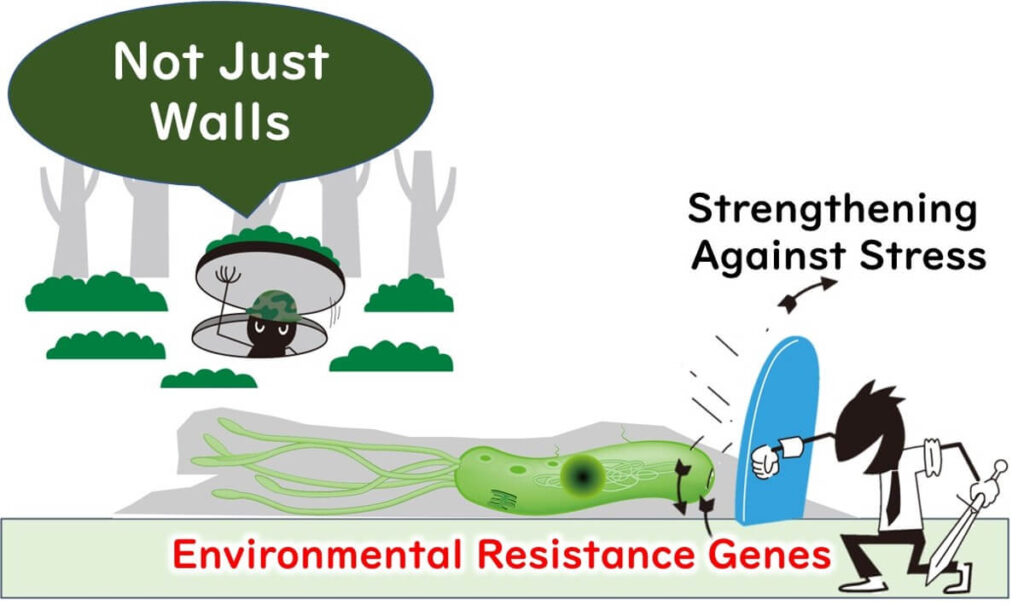
Where Biofilms are Likely to Form
Biofilms can form in many hidden and often overlooked areas of food factories. Identifying these high-risk locations is crucial for maintaining hygiene and preventing contamination:
Beneath large equipment: These areas are often missed during routine cleaning and provide an ideal environment for biofilm formation.

Inside and outside of pipes: These are notoriously difficult to clean and prone to biofilm development.
Undersides of factory floor mats: Often neglected, these areas can harbor biofilms and contribute to contamination.

Trolley wheels: Used for transporting goods, these wheels are hard to clean and can spread biofilm-related microbes across the factory.
Faucets: While sinks are cleaned regularly, the faucets themselves are frequently touched by workers and can easily accumulate biofilms.
Cleaning tools: Items like sponges, brushes, and gloves can inadvertently spread biofilms if not properly maintained.

Control panels and buttons: Frequently touched by workers, these surfaces are often overlooked in cleaning routines.
Stainless steel surfaces: Even minor scratches on these surfaces can trap organic matter, creating conditions favorable for biofilm formation.
By regularly inspecting and cleaning these areas, factories can significantly reduce the risk of biofilm-related contamination.
Biofilm Removal Methods
Effectively removing biofilms requires a targeted approach, as there are no shortcuts or special sterilization techniques designed exclusively for biofilms. The following steps are key:
- Thorough Cleaning: The first step is to identify biofilm-prone areas and treat them as sources of organic dirt.
- Physical Removal: Physically scrubbing surfaces to eliminate biofilm layers is essential. Mechanical cleaning can dislodge the stubborn layers of cells and organic matter.
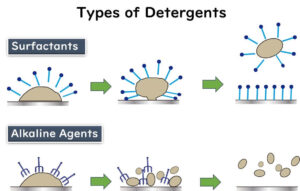
- Chemical Cleaning: Use detergents and cleaning agents appropriate for the type of organic matter present, such as lipids or proteins. Tailor the cleaning agents to the specific contaminants found in the factory environment.
- Sterilization Only After Cleaning: Attempting to sterilize biofilms without first cleaning the surface is ineffective. Cleaning must precede sterilization to ensure the disinfectant can reach and eliminate all microbes.
For further insights into cleaning and sterilization in food factories, refer to the related article:
Disinfection vs. Cleaning in Food Safety: Why Thorough Cleaning is the Foundation