Ever had a dodgy chicken sandwich that left your stomach in knots? Or heard of someone feeling numbness after a bout of food poisoning? It might have been down to a little mischief-maker called Campylobacter. In this post, we'll dive into what this bacterium is all about, why it loves to hide in chicken, and some of its peculiar habits like thriving in warm environments and being a bit shy around oxygen. Plus, we'll discuss a mysterious condition called Guillain-Barré Syndrome that might follow this food poisoning – a syndrome where your body's own defense system goes a bit rogue. And, of course, we'll give you some top tips to keep these unwanted dinner guests away from your plate. Ready to become a food safety expert? Let's dive in!
Understanding Campylobacter: A Domino Effect from its Habitat
Before we dive into the details of Campylobacter, it might be helpful to first read our foundational article on Gram staining and microbial characteristics available on our blog. This background information will create a smooth flow for understanding Campylobacter, like a well-aligned row of dominos.
Gram Staining and Microbial Properties: A Comprehensive Overview
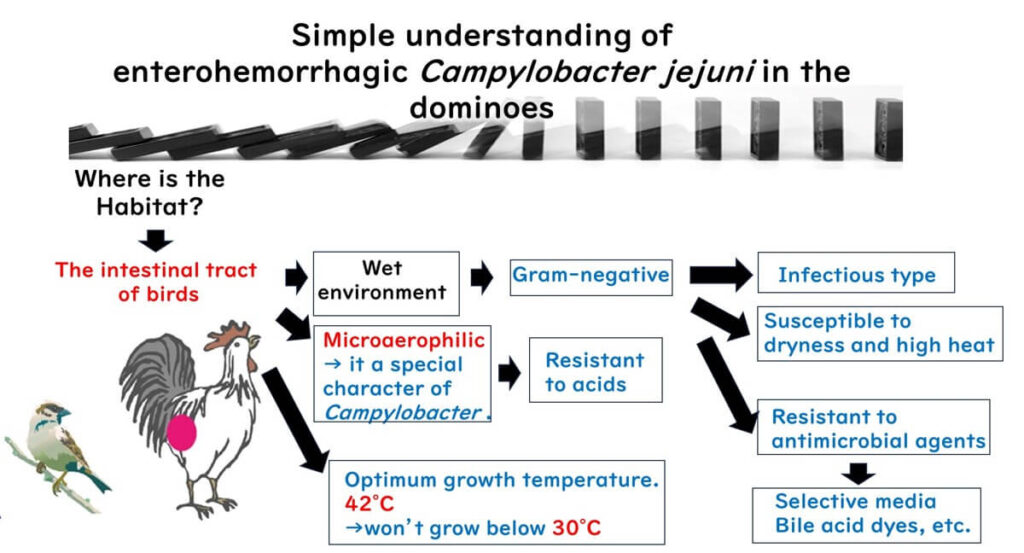
- Habitat of Campylobacter: This bacterium primarily inhabits the intestines of birds, which provide a moist environment.
- Type of Bacterium: Given its habitat, we understand that Campylobacter is a Gram-negative bacterium.
- Infection Nature: As a Gram-negative bacterium, Campylobacter is capable of causing infectious food poisoning.
- Unique Growth Temperature: What sets Campylobacter apart is its optimal growth temperature of 42°C, and its inability to grow below 30°C.
- Sensitivity to Conditions: Like other Gram-negative bacteria, Campylobacter is vulnerable to drying and high heat.
- Oxygen Requirements: Unlike many bacteria, Campylobacter doesn’t thrive in standard oxygen levels (20%) or complete oxygen absence. Instead, it grows best in environments with 3–15% oxygen, making it a “microaerophilic” bacterium. Unlike facultative anaerobes, which can switch metabolic processes depending on oxygen availability, Campylobacter requires moderate oxygen levels to sustain its energy production through the citric acid cycle.Campylobacter can be thought of as a bacterium that “needs sunlight but is easily sunburned.” It thrives with a specific oxygen range, but too much exposure is harmful.
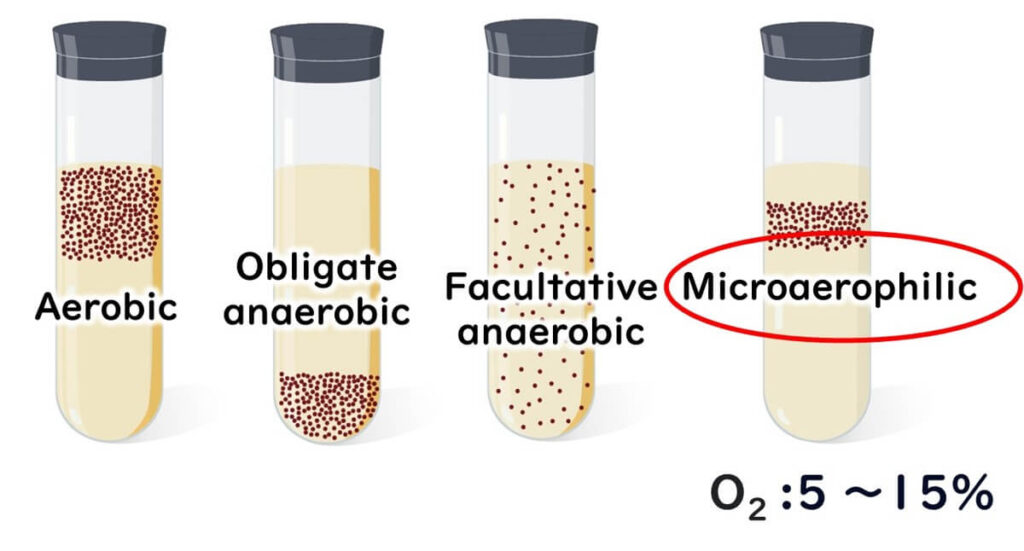
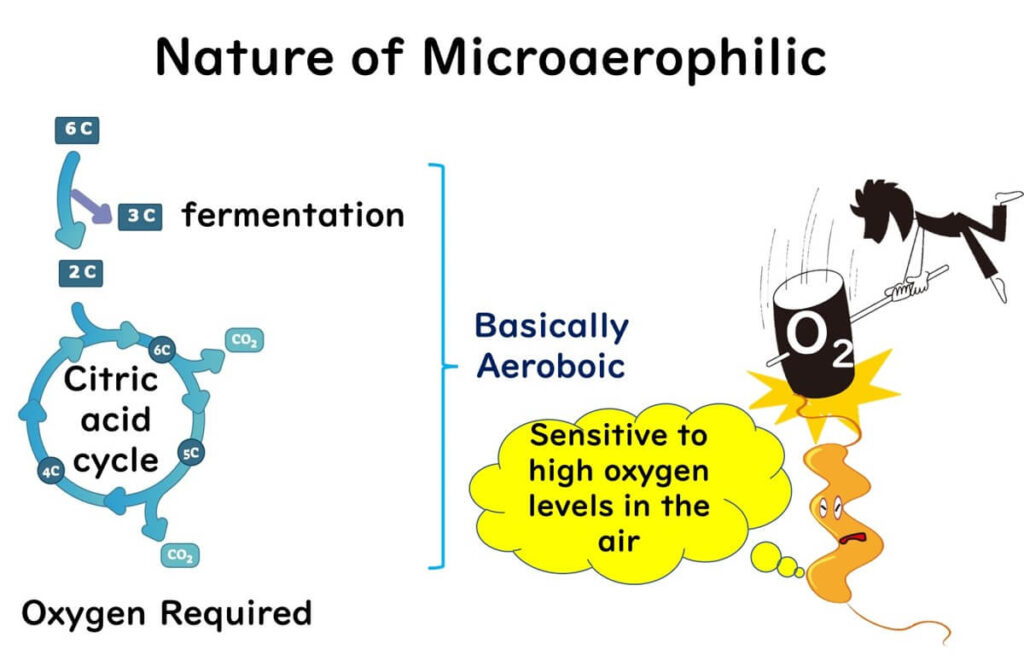
For more on the difference between anaerobic and aerobic bacteria, see our article below:
Understanding Gram-Negative Bacteria: The Role of the OF Test
- Growth during Chicken Distribution: Because its minimum growth temperature is 30°C, Campylobacter does not multiply in standard storage temperatures used for chicken or chicken products.
- Laboratory Growth of Campylobacter: Being Gram-negative, Campylobacter can be selectively cultured using compounds that inhibit Gram-positive bacteria.
By understanding these points sequentially, as in a domino effect, we can better comprehend Campylobacter’s characteristics and its role in food safety.
The Spiral Shape of Campylobacter
Bacteria come in various shapes, but the two most common forms are spherical (coccus) and rod-shaped (bacillus). Campylobacter, however, stands out with its unique spiral or helical shape, unlike most bacteria.
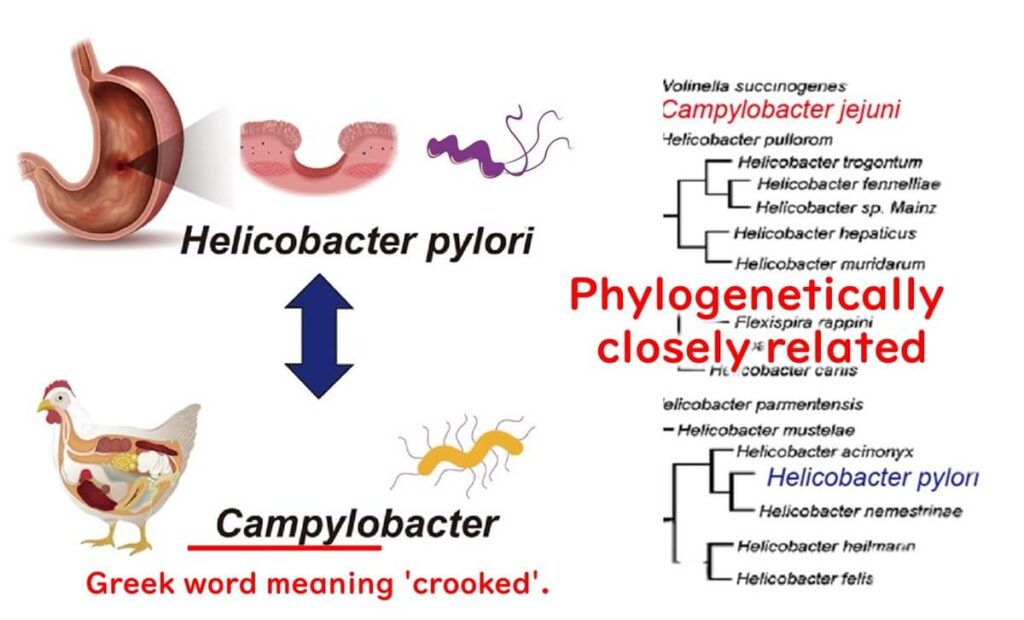
This corkscrew-like form is rare among bacteria. Campylobacter shares this spiral shape with another well-known bacterium, Helicobacter pylori, which is associated with stomach ulcers.
Interestingly, there was a time when scientists believed that Helicobacter pylori was a type of Campylobacter. However, advances in molecular biology, particularly with the analysis of 16S ribosomal DNA, revealed that these two bacteria belong to different genera. It’s a fascinating twist in the story of microbiology!
The Natural Home for Campylobacter
It's widely believed that the primary natural hosts for Campylobacter are birds. Let’s explore why:
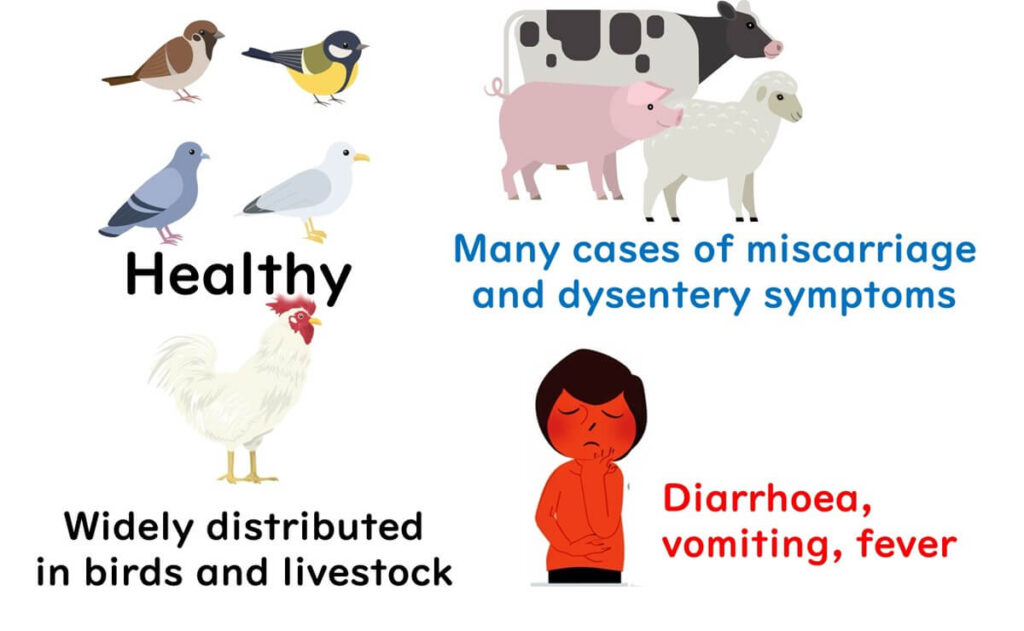
Widespread Presence in Birds
Campylobacter is commonly found in the gastrointestinal tracts of various bird species in the wild. Birds carry Campylobacter without showing any symptoms, while in pigs and cows, the bacteria might cause adverse effects, like miscarriages or dysentery.
The Temperature Connection
Campylobacter thrives at a toasty 40-42°C, which aligns with the body temperature of birds. Birds need to maintain this higher body temperature—around 5°C above that of mammals—to stay active and counter rapid heat loss.
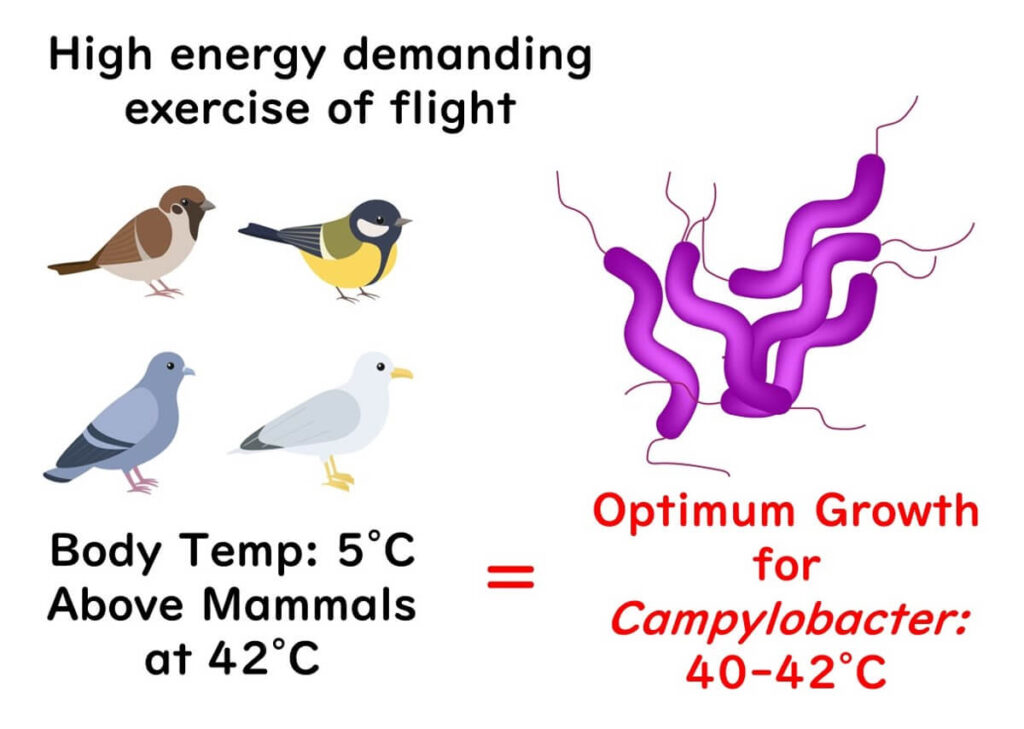
Given these reasons, it’s no surprise that the optimal temperature for Campylobacter matches the body temperature of birds. Looking at all the evidence, it's reasonable to say birds are the natural hosts for Campylobacter. Cool, right?
The Culprit Behind Campylobacter Gastroenteritis: Chicken Meat
When we talk about Campylobacter gastroenteritis, chicken is often the primary suspect. Most people don’t consume raw chicken, so cases of Campylobacter food poisoning typically result from undercooked dishes, like fried or grilled chicken. However, the risk doesn’t stop at cooking. Imagine this scenario: you chop raw chicken with a knife, and then use the same knife or cutting board on another dish without proper cleaning. This cross-contamination can easily lead to foodborne illness.
In Japan, some people do enjoy eating raw chicken, which has led to Campylobacter being a primary cause of food poisoning there.
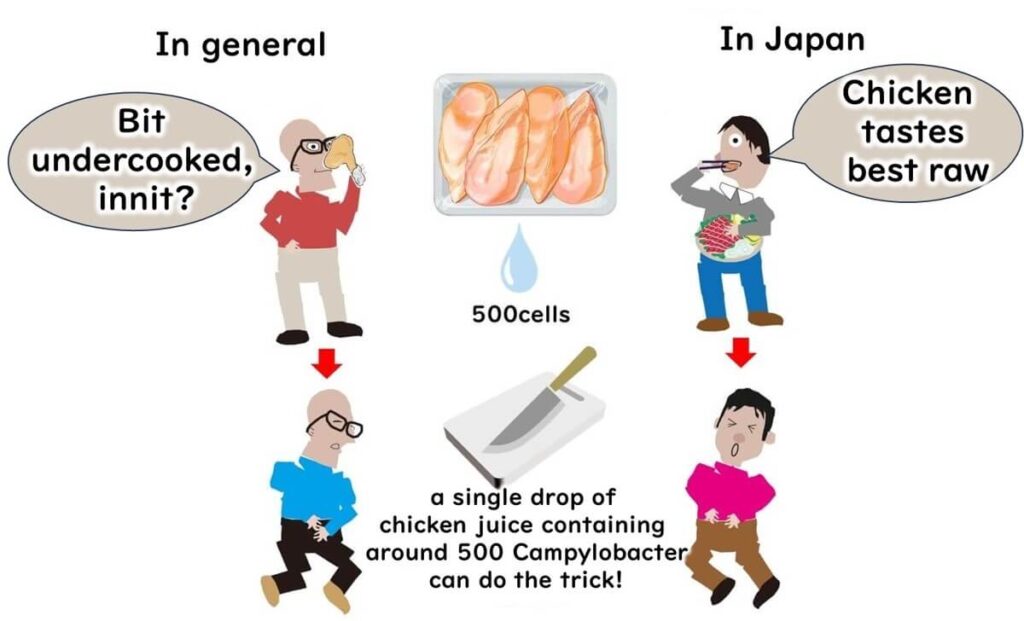
Interestingly, once Campylobacter is transferred from a contaminated surface like a knife, it doesn’t multiply on other foods. This is because it requires a minimum temperature of 30°C to grow. However, Campylobacter, closely related to the stomach-acid-resistant Helicobacter pylori, can withstand stomach acid, making it particularly infectious. In fact, as few as 500 bacterial cells—equivalent to a drop of chicken juice—can cause illness.
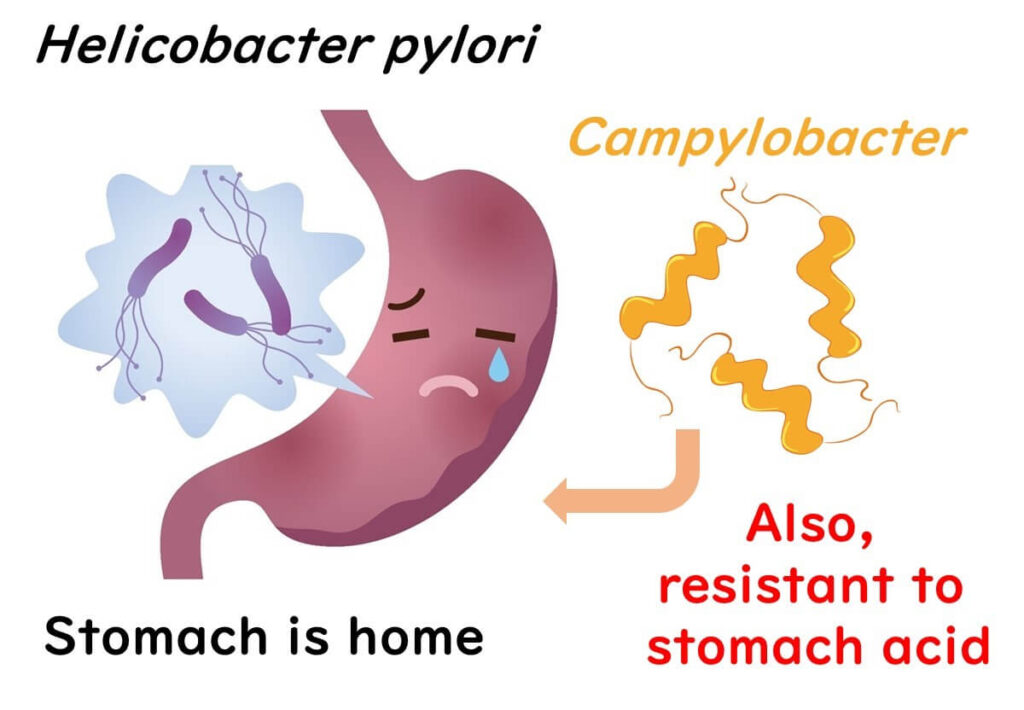
So, secondary contamination from tools and surfaces plays a significant role in Campylobacter gastroenteritis, even in cases where bacteria numbers are low. Globally, whether through raw or undercooked chicken or cross-contamination, Campylobacter on chicken remains a common culprit in foodborne illness.

Why It Takes So Long to Get Sick from Campylobacter
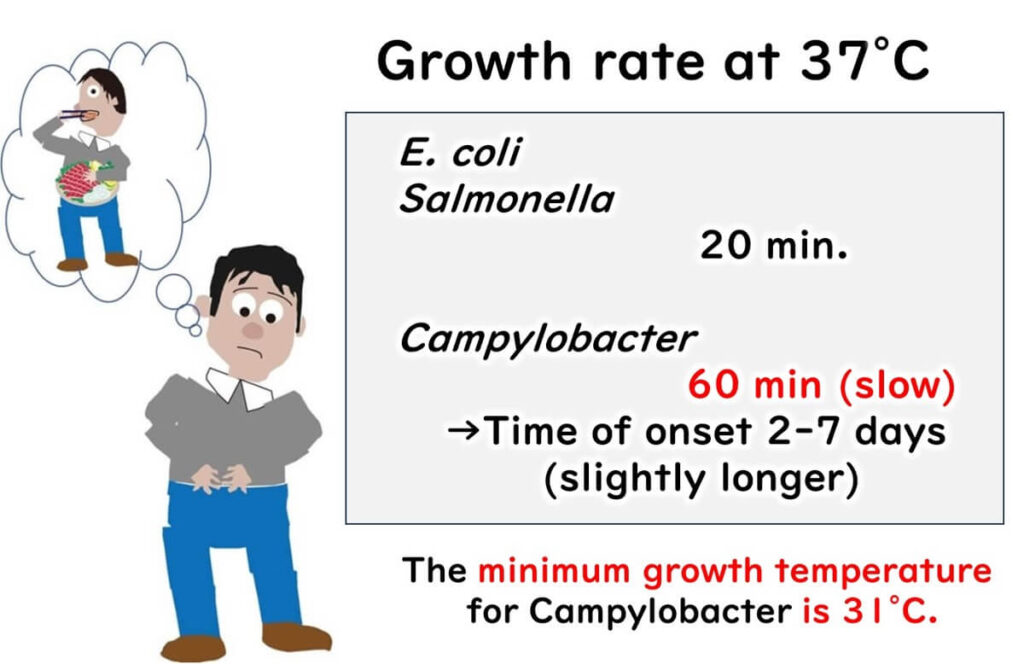
One tricky aspect of Campylobacter gastroenteritis is its delayed onset. Unlike infections caused by Salmonella or E. coli, which typically result in symptoms like bloody diarrhea within about 24 hours, Campylobacter can be sneaky. Symptoms may not appear until 2 to 7 days after consuming contaminated food.
Why the delay? Bacteria like Salmonella and E. coli multiply rapidly at body temperature, doubling every 20 to 30 minutes. But Campylobacter is slower—it takes a full hour to divide at 37°C. Below 30°C, it can’t divide at all.
Due to this slower growth rate and higher temperature requirement, it can take longer for Campylobacter to reach levels that trigger illness. This lag time means Campylobacter gastroenteritis symptoms might not appear until well after the initial exposure. It’s a long wait that nobody wants, but it’s a characteristic that makes Campylobacter distinct from other foodborne pathogens.
Why Campylobacter Might Be Sneakier Than You Think
When Campylobacter infects the cells lining our intestines, it disrupts normal absorption and secretion processes, leading to an increased release of fluids and, ultimately, diarrhea.
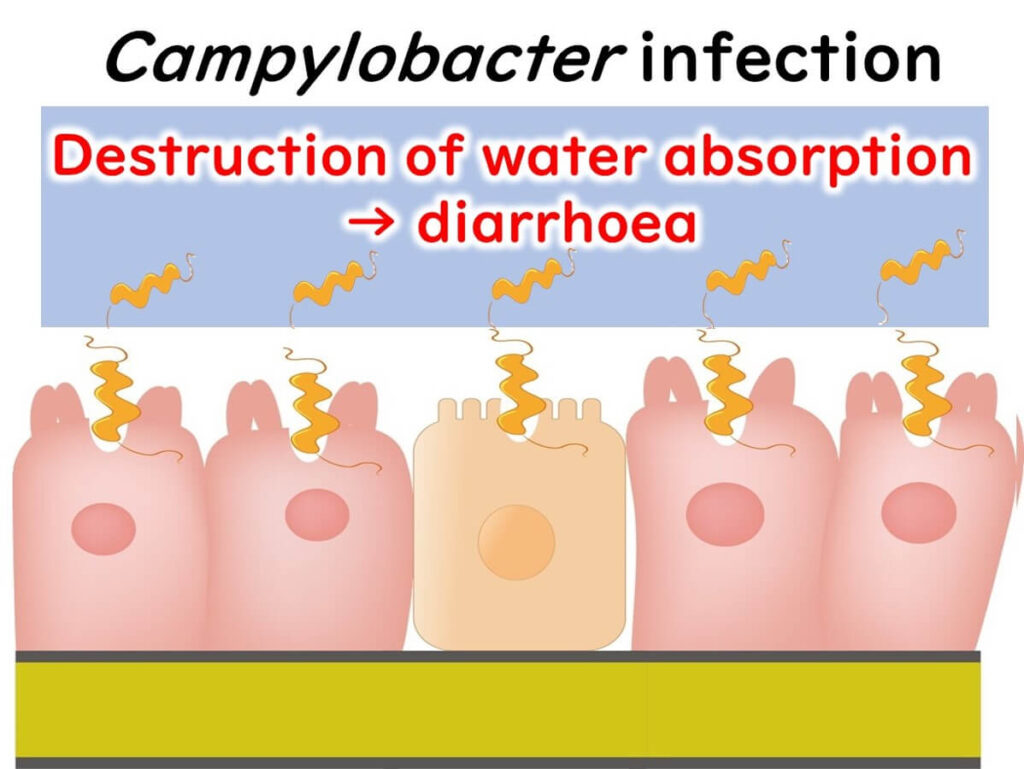
nitially, symptoms from Campylobacter poisoning might not seem severe, and most people recover within 2 to 5 days.

But here’s the twist: about 10 days after recovery, approximately 1 in 1,000 people may develop Guillain-Barré Syndrome (GBS), a condition where nerves in the arms and legs become paralyzed, potentially affecting movement, walking, and even breathing in severe cases.
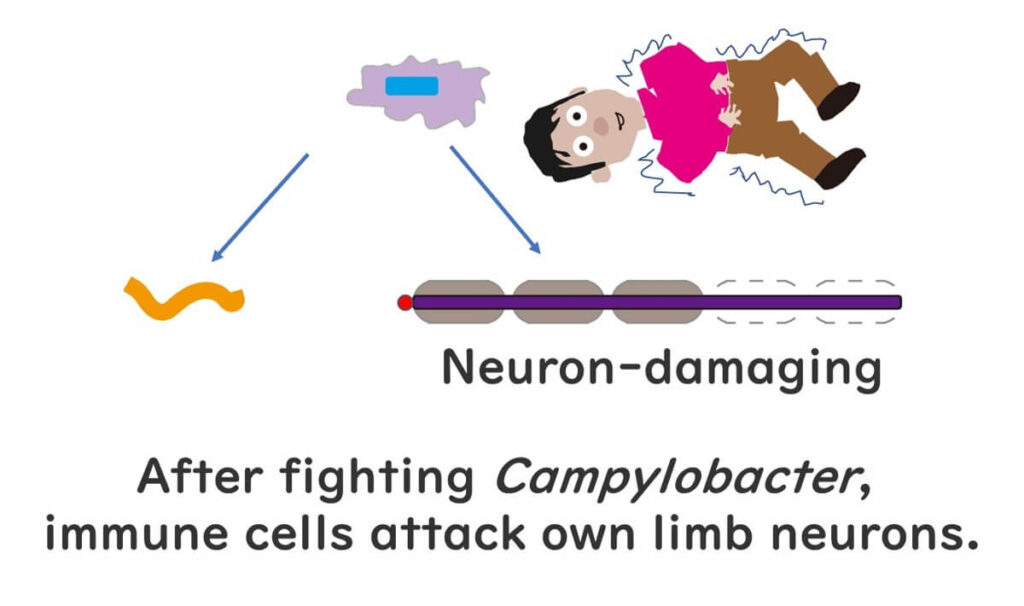
Why does this happen after a Campylobacter infection? Scientists believe that GBS is triggered when our immune system mistakenly targets our own nerve cells. This misidentification occurs because certain sugars (polysaccharides) on our nerve cells resemble those found on the outer layer of Campylobacter. When we’re infected, our immune system goes into action to fight Campylobacter by targeting these sugars. Although the immune cells successfully fend off the bacteria, they sometimes remain activated and misidentify our own cells as foreign, leading to GBS.
It’s fascinating—and a bit alarming—how such a small bacterium can have such significant effects on our bodies, highlighting the complex interplay between pathogens and our immune system.
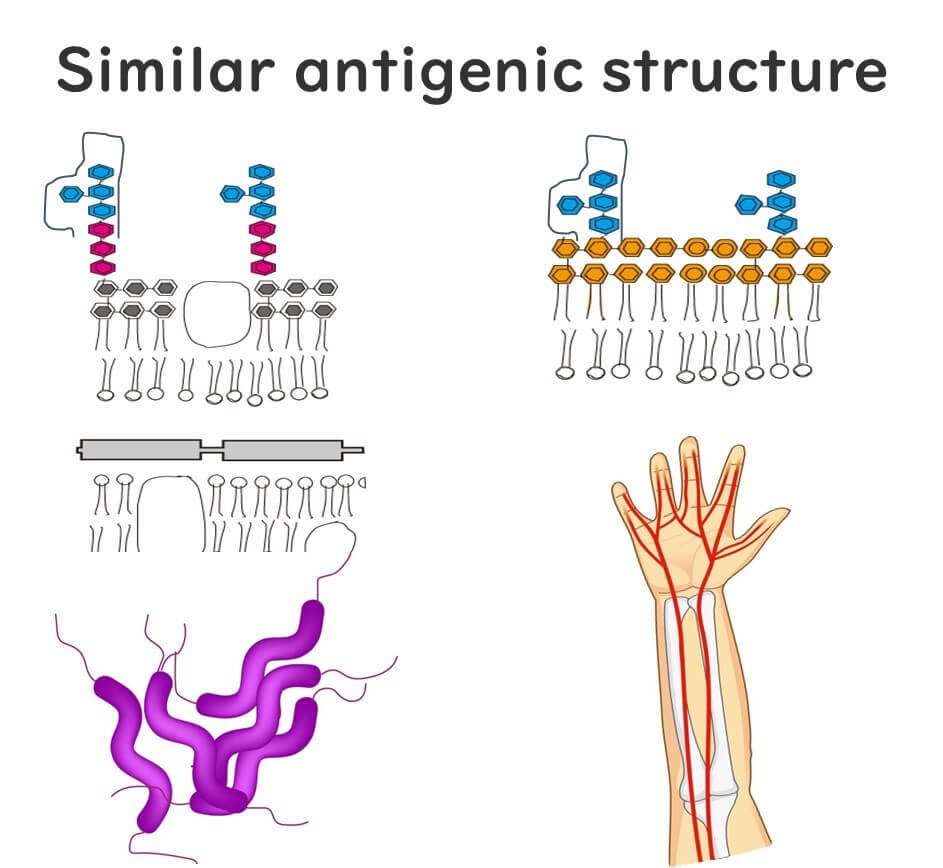
Guillain-Barré Syndrome: Not Just After Food Poisoning, But COVID-19 Too?
Remember Guillain-Barré Syndrome (GBS), the condition that can cause paralysis in the arms and legs after a Campylobacter infection? It turns out that food poisoning isn’t the only potential trigger for GBS. Other infections, such as the flu, have also been linked to this syndrome, with Campylobacter being the most commonly reported precursor.
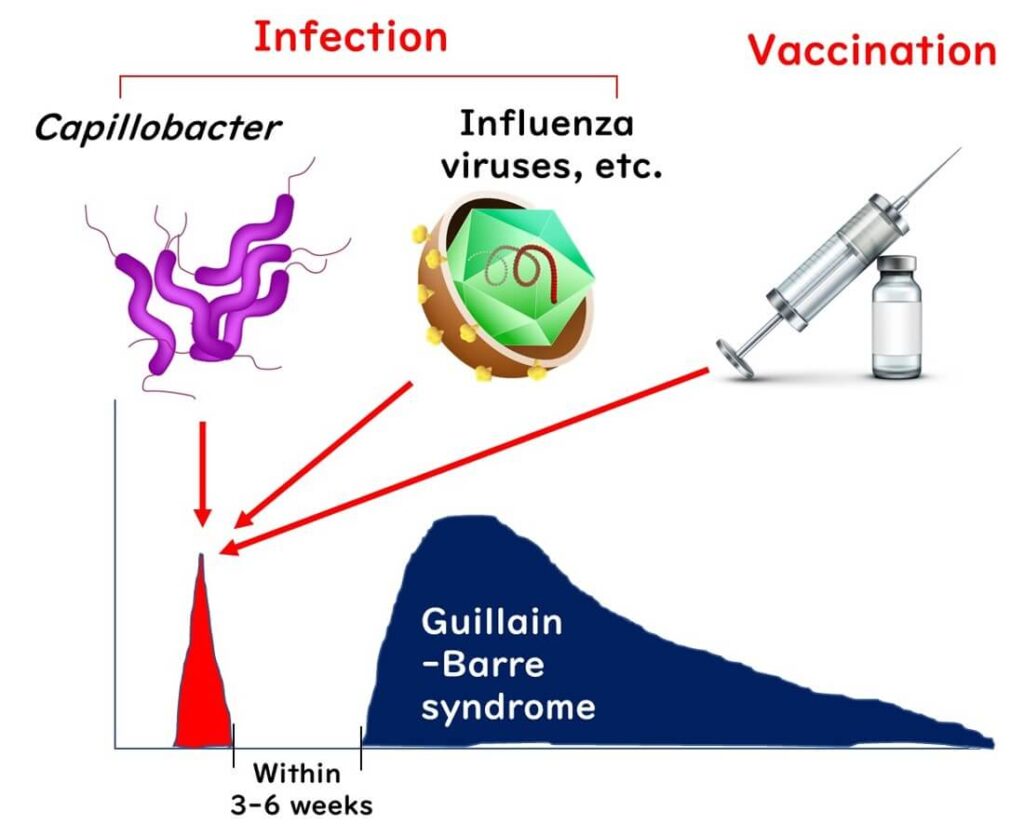
Recently, there have been reports suggesting that COVID-19 might also be linked to GBS in some cases. Ongoing studies continue to investigate this possible connection, as researchers examine the wider effects of viral infections on our immune system and their potential to trigger conditions like GBS.
IThis surprising link between foodborne and respiratory infections like COVID-19 reminds us of the complex and interconnected relationship between our immune responses and various pathogens.
Food Distribution & Campylobacter
When it comes to foodborne bacteria, Campylobacter stands out for several reasons. First, it thrives in warmer environments—about 42°C, which is higher than the optimal growth temperature of most foodborne pathogens. However, Campylobacter won’t grow at temperatures below 30°C, meaning it doesn’t proliferate even if chicken is left out at room temperature. This sets it apart from bacteria like Salmonella and E. coli, which can grow at temperatures as low as 10°C.

Even if there’s a lapse in temperature control during chicken distribution, Campylobacter won’t multiply. Moreover, as a "microaerophilic" bacterium, it doesn't grow well on most foods, including chicken. So, keeping chicken refrigerated or controlling its temperature won’t directly affect Campylobacter levels. Instead, the focus needs to be on managing the initial contamination levels at chicken farms and processing facilities.
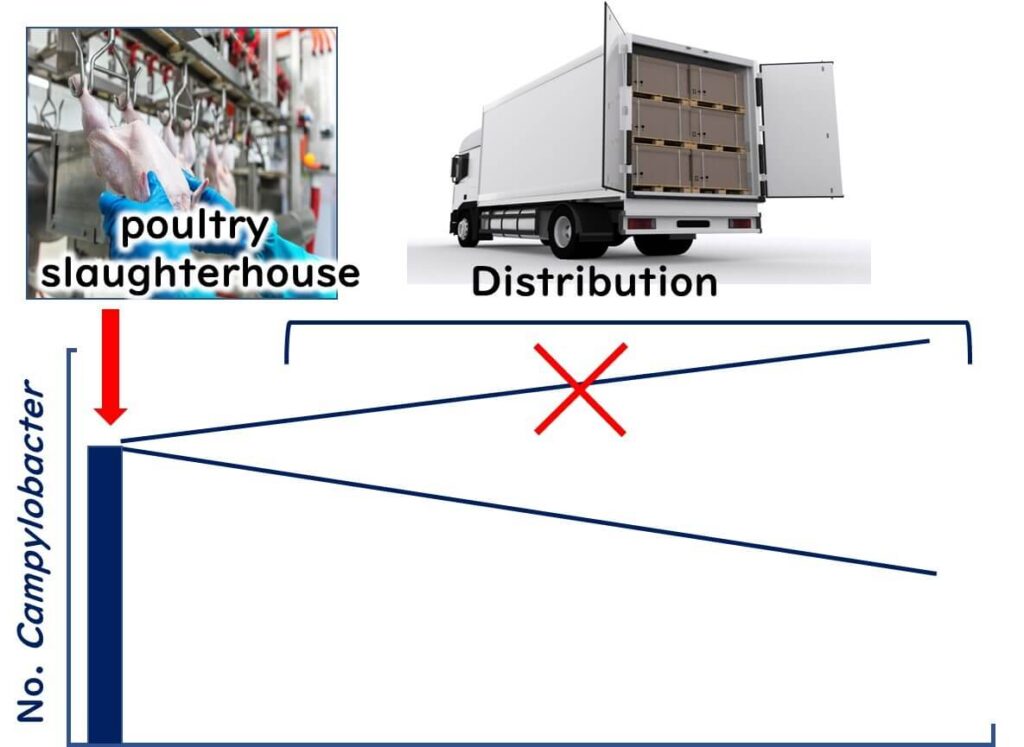
In the U.S., poultry processing plants often use sodium hypochlorite to reduce Campylobacter contamination. Newer methods combine peracetic acid with organic acids for potentially more effective results. The EU, however, bans the use of such chemical treatments in poultry processing. They argue that strong disinfectants may mask hygiene issues in the earlier stages of the supply chain.
This insight into Campylobacter highlights the unique challenges in controlling its spread and the importance of comprehensive hygiene management across the entire food processing system.