In the world of ethanol sterilization, 70% concentration is the gold standard. But why is this specific ratio so effective against microbes, and why does it fall short against challenges like norovirus? While some uncertainties remain about the exact mechanisms, this article explores the science behind ethanol's germ-killing power and its limitations, offering a closer look at its role in effective microbial control.
Why 70% Ethanol is the Ideal Concentration for Microbial Control
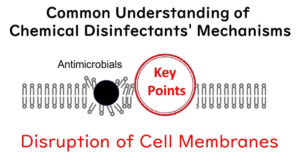
So, let's dive into the murky waters of scientific speculation, shall we? The consensus from numerous lab showdowns (a.k.a. experiments) suggests that when microbial cells are given a 70% alcohol bath, they end up spilling their guts—literally.
Under the microscope, it's like watching a cell membrane go "pop!" leading to the contents being laid bare. So, it's believed that alcohol's main gig is to wreak havoc on the cell membrane.
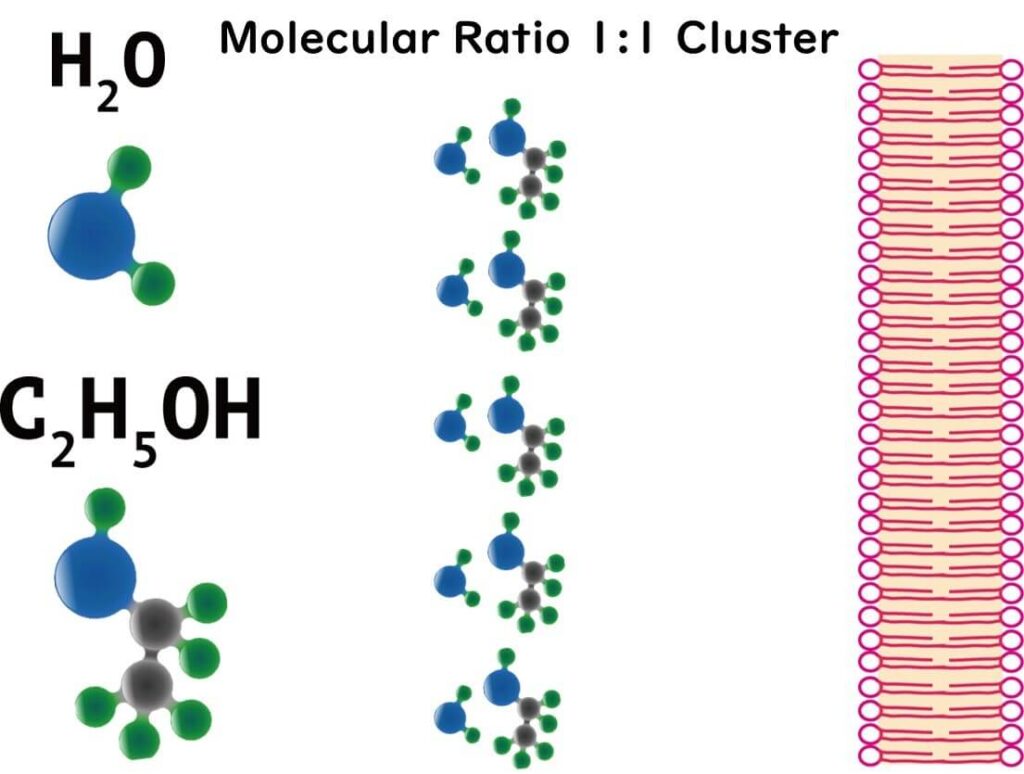
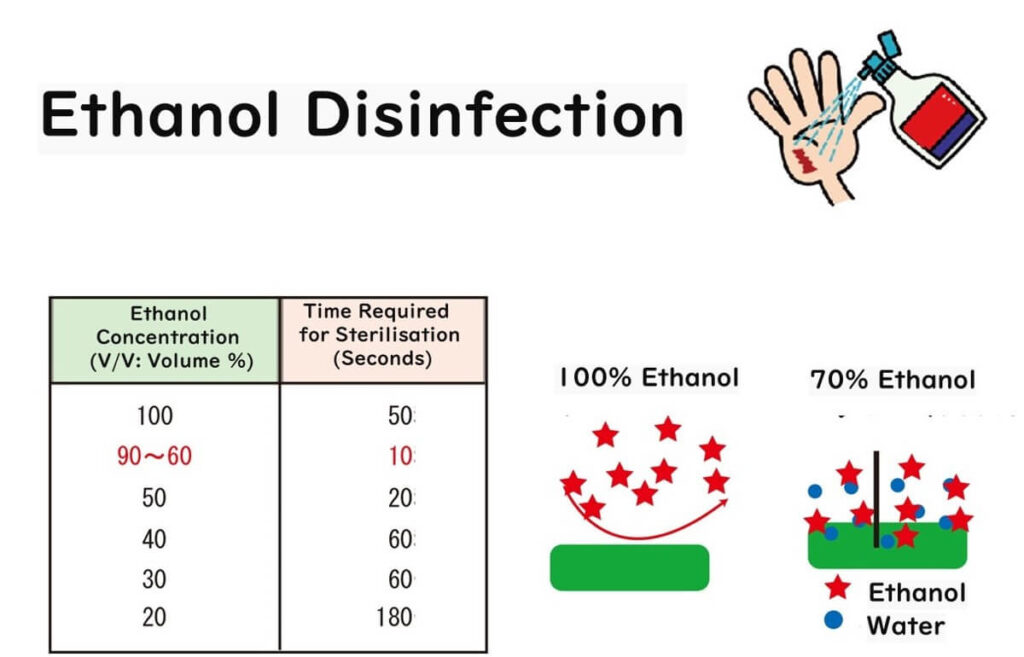
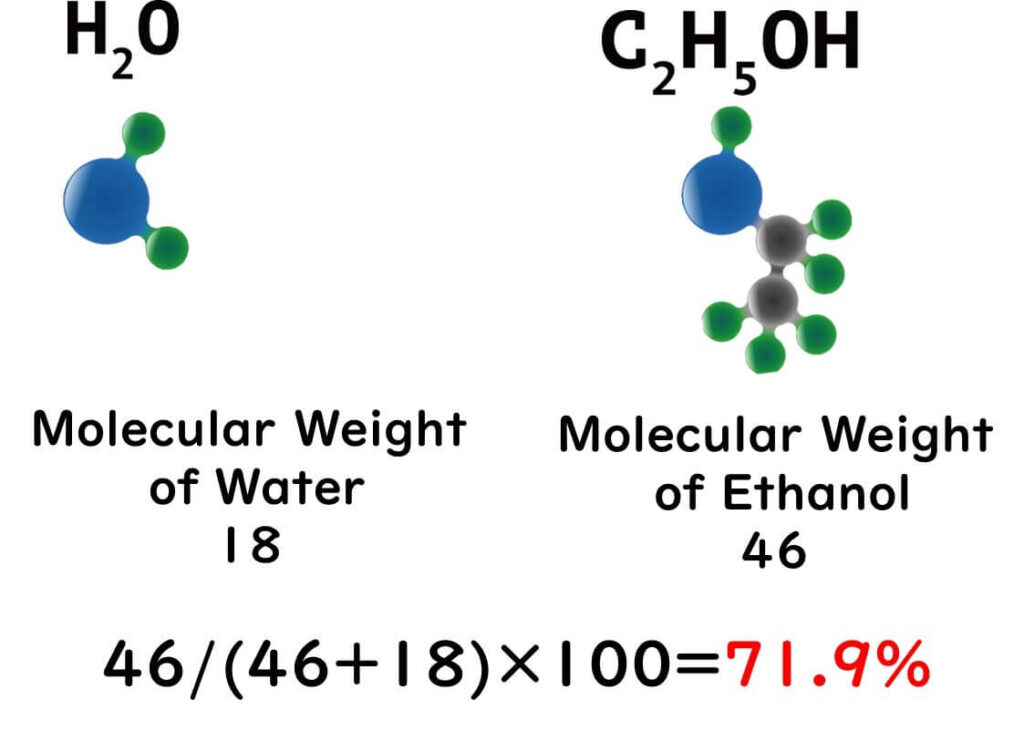
But here's where it gets science-y: in an aqueous solution, ethanol molecules form a sort of conga line with water molecules, creating what's known as polymer structures. At a cosy 1:1 ratio, which happens to be the case with our 70% solution, ethanol and water molecules form the most stable clusters. These clusters, with their massive hydrophobic exteriors, are thought to be the cell membrane's worst nightmare.
This unique balance between ethanol and water enhances the penetration and effectiveness of the solution, making 70% ethanol the gold standard for sterilization in laboratories and food safety practices.
Why Ethanol Fails Against Norovirus Despite Its Success With Other Viruses
Now, onto the Achilles' heel of ethanol sterilization—its faceplant when up against norovirus. To understand this, we need to talk about two types of viruses.
On one hand, we have the likes of COVID-19 and influenza viruses, which are pretty clever in their infection strategy. These envelope viruses exit their host cell wrapped in a piece of the host's own membrane. This sneaky exit strategy helps them blend in and infect more efficiently.
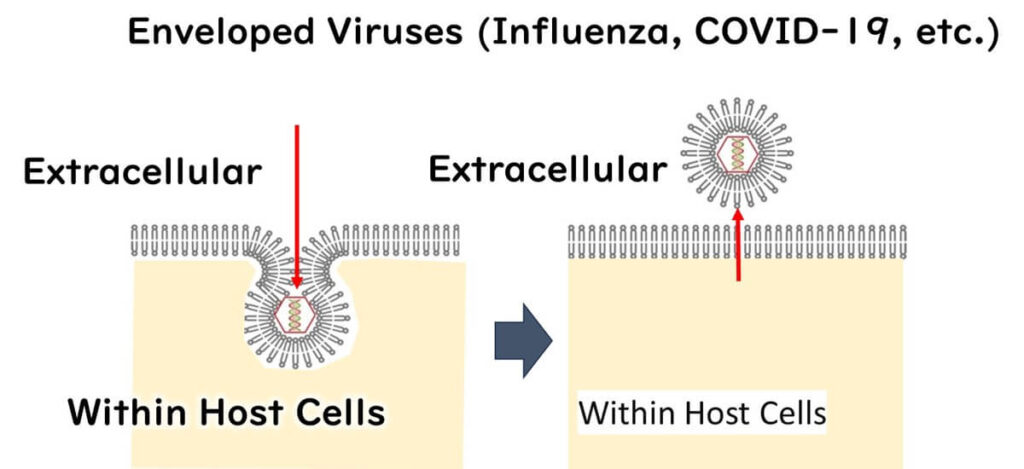
However, their reliance on a lipid envelope is also their weakness. This delicate layer makes them vulnerable to environmental stressors, including ethanol, which disrupts the lipid structure and effectively inactivates the virus.

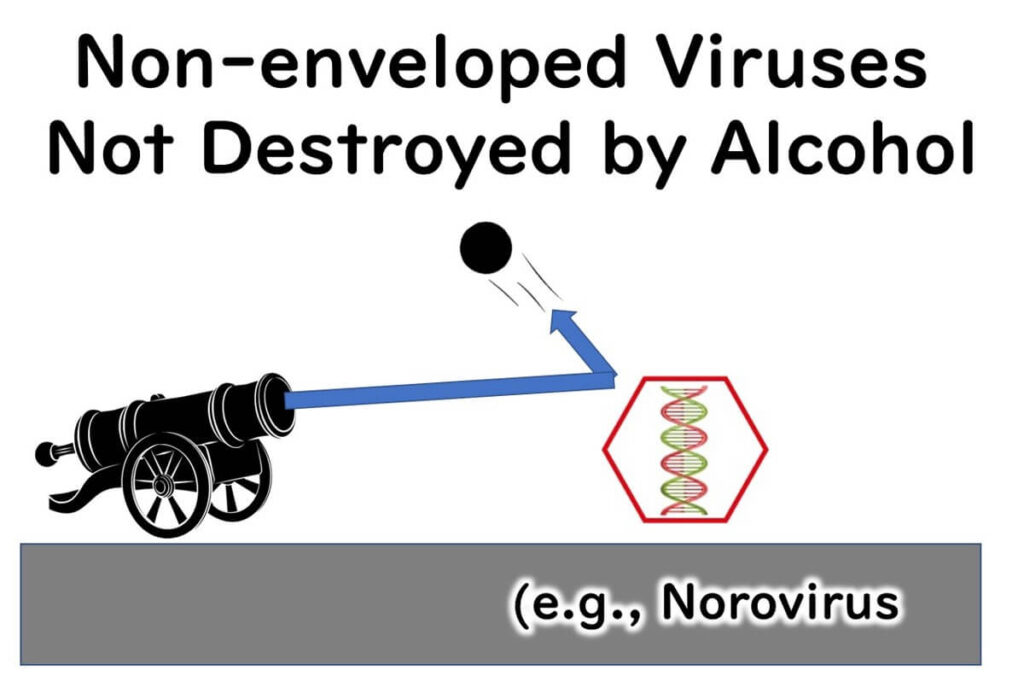
In contrast, norovirus lacks a lipid envelope and consists only of RNA enclosed in a robust protein capsid. This simple yet durable structure allows norovirus to resist environmental stress and renders it highly resistant to alcohol-based disinfectants.
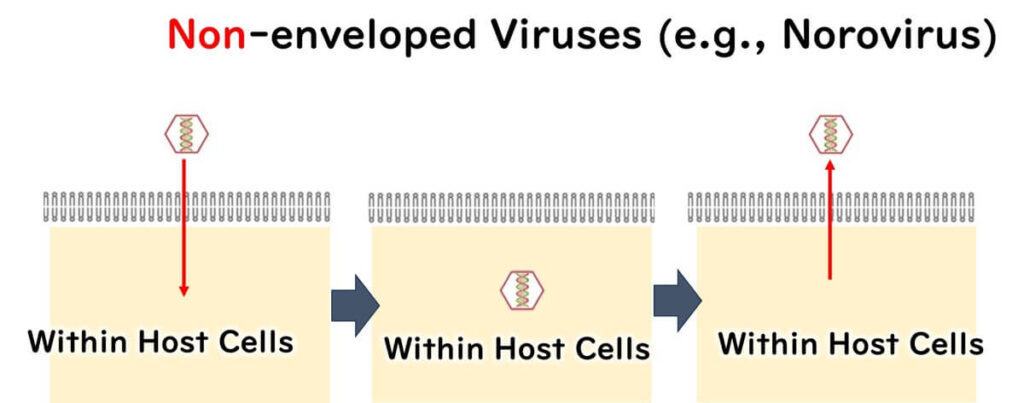
This explains why ethanol is highly effective against enveloped viruses like COVID-19 and influenza but has little impact on norovirus. These differences underscore the importance of choosing disinfectants that are specifically suited to the characteristics of the target pathogen.