In the heart of every food factory lies a relentless battle against grime and germs. To ensure pristine conditions and uphold food safety standards, cleaning and disinfecting agents are the unsung heroes, working tirelessly to maintain a safe and hygienic environment. Let’s delve into their crucial role in safeguarding food quality.
Let’s explore the fascinating world of cleaning agents and disinfectants in food factories. Imagine a microscopic battlefield, where grime and germs are the relentless adversaries. Our heroes? The cleaning and disinfecting agents, each equipped with unique powers to ensure cleanliness and food safety. Let’s see how they work.
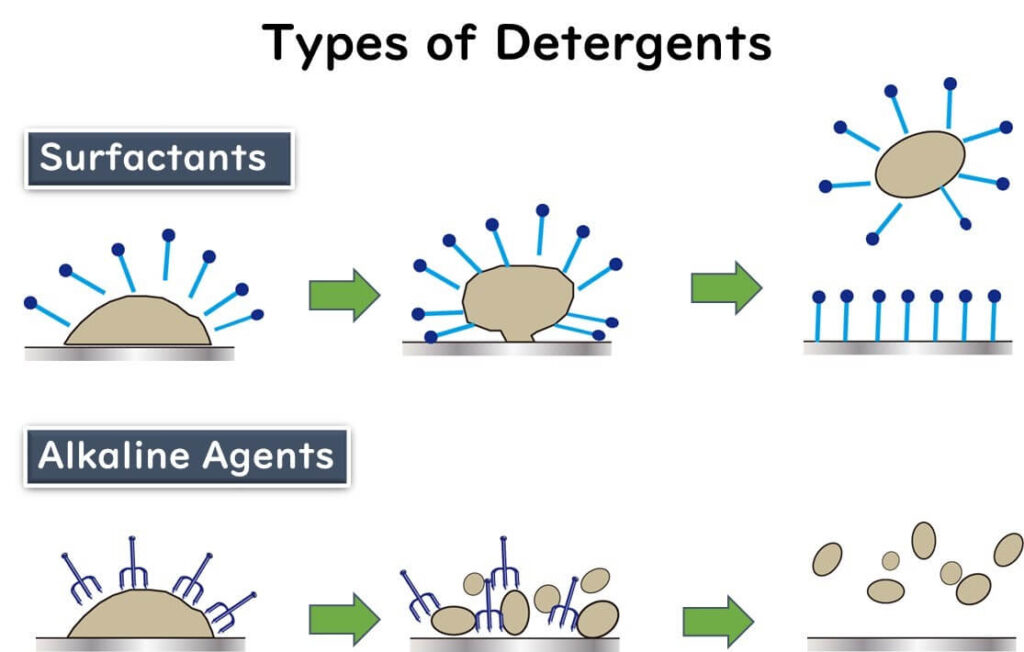
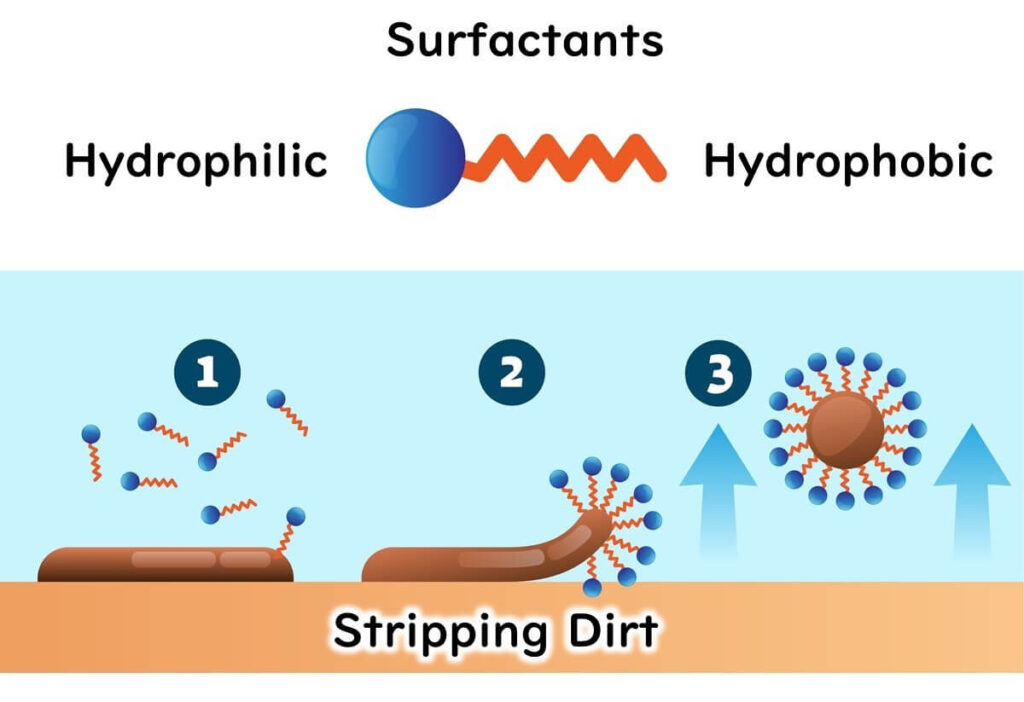
Surfactants: The Key to Tackling Oily Residues
Surfactants are like the double agents in spy movies. With their hydrophilic (water-loving) head and hydrophobic (oil-loving) tail, these molecules excel at breaking through oily residues commonly found in food processing environments. They work by surrounding dirt and grease on surfaces, lifting them away like a helicopter rescuing stranded debris. This action is critical for cleaning machinery and workstations effectively, setting the stage for proper disinfection.
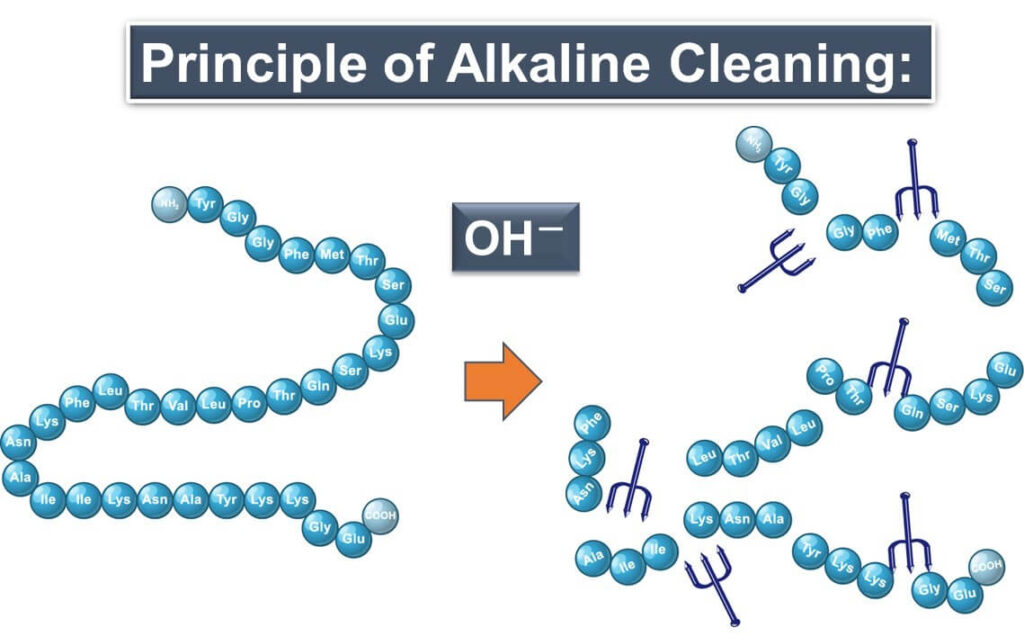
Alkaline Cleaners: Breaking Down Stubborn Contaminants
Alkaline cleaners are the powerhouses of cleaning agents. Packed with OH- ions, they don't just remove dirt; they break it down at a molecular level. These ions attack proteins and other organic residues, reducing them to smaller peptides or fragments. This process is essential for removing protein-based contaminants like meat juices or dairy residues, ensuring a thorough clean and a safe environment for food processing.