Hello everyone! In this article, we’ll uncover a fascinating aspect of microbiology: how the Catalase Test helps differentiate lactic acid bacteria from other Gram-positive microorganisms. Unlike their Gram-positive counterparts, lactic acid bacteria don’t produce oxygen bubbles during this test. We’ll also explore how they adapt to oxygen-rich environments despite this unique trait. Curious to learn more? Let’s dive in!
Identifying Lactic Acid Bacteria with the Catalase Test
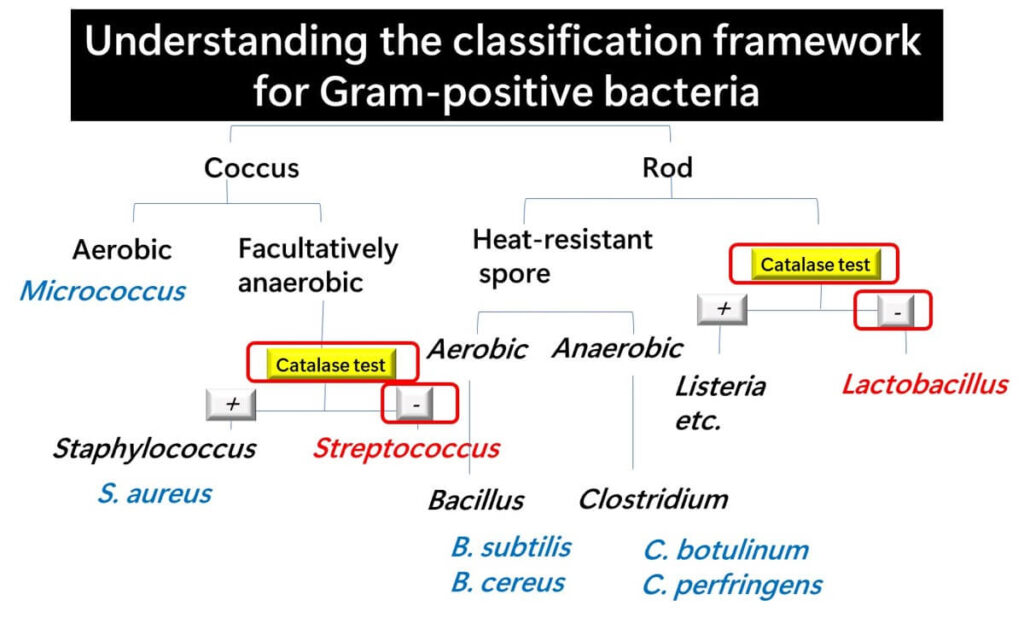
To begin, it’s essential to understand that lactic acid bacteria belong to the Gram-positive group. The Catalase Test is an effective tool for distinguishing them from other bacteria within this group.
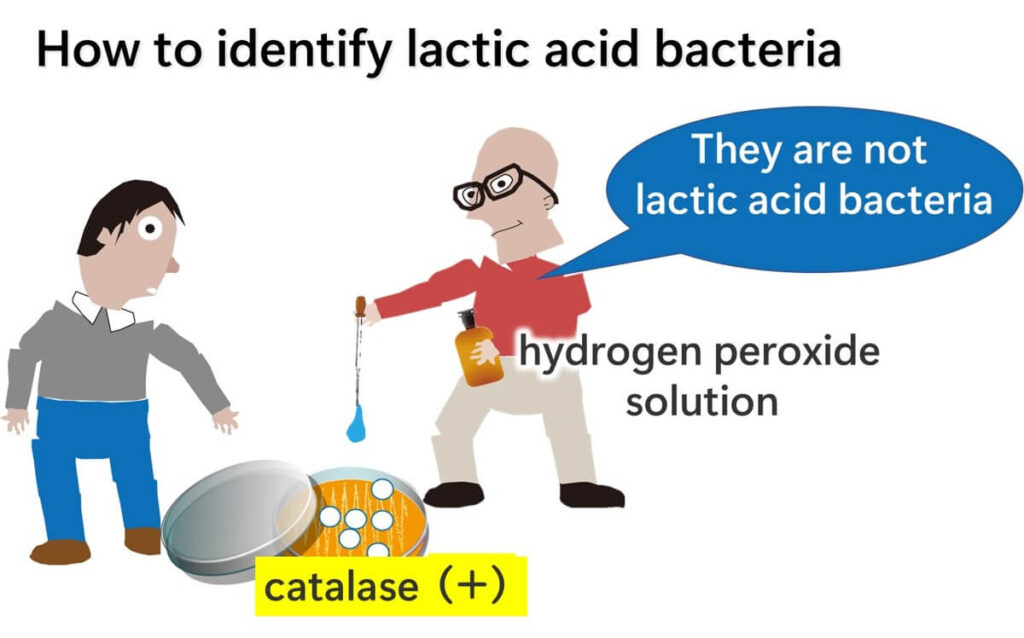
Here’s how the test works: place a drop of hydrogen peroxide onto a bacterial colony. If bubbles form around the colony, the result is positive, indicating the presence of the catalase enzyme.
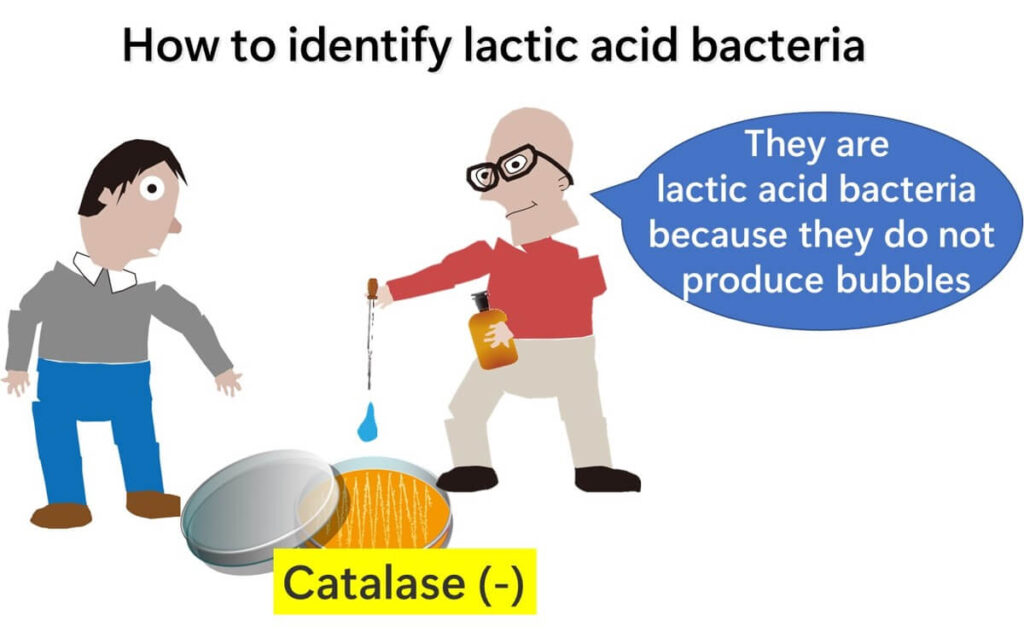
Most organisms that thrive in oxygen-rich environments produce catalase. When performing this test on a standard agar plate, bubbles are typically observed. However, if no bubbles form and the colony is Gram-positive, it can be confidently identified as lactic acid bacteria, regardless of whether its shape is spherical (coccus) or rod-shaped (bacillus).
Note: This test is applicable to colonies grown in aerobic (oxygen-present) environments and does not apply to those requiring anaerobic (oxygen-free) conditions.
Catalase Enzyme in Microbiology
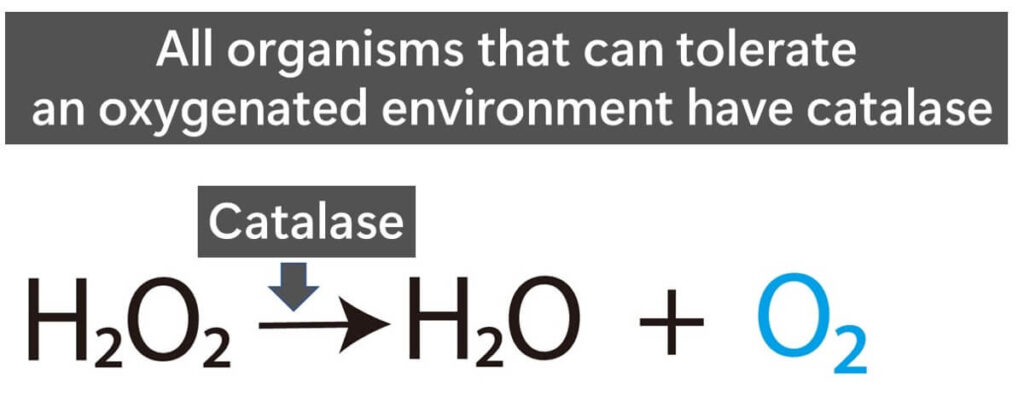
So, what is catalase? Catalase is an enzyme that breaks down hydrogen peroxide into water and oxygen. Its primary function is to protect cells from the harmful effects of hydrogen peroxide, a toxic by-product of metabolic processes when allowed to accumulate.

In Earth's early history, oxygen was virtually absent. It later appeared as a by-product of photosynthesis performed by cyanobacteria. This development drastically altered the Earth’s environment, often referred to as the first environmental crisis, as many primitive life forms struggled to adapt to the presence of oxygen.
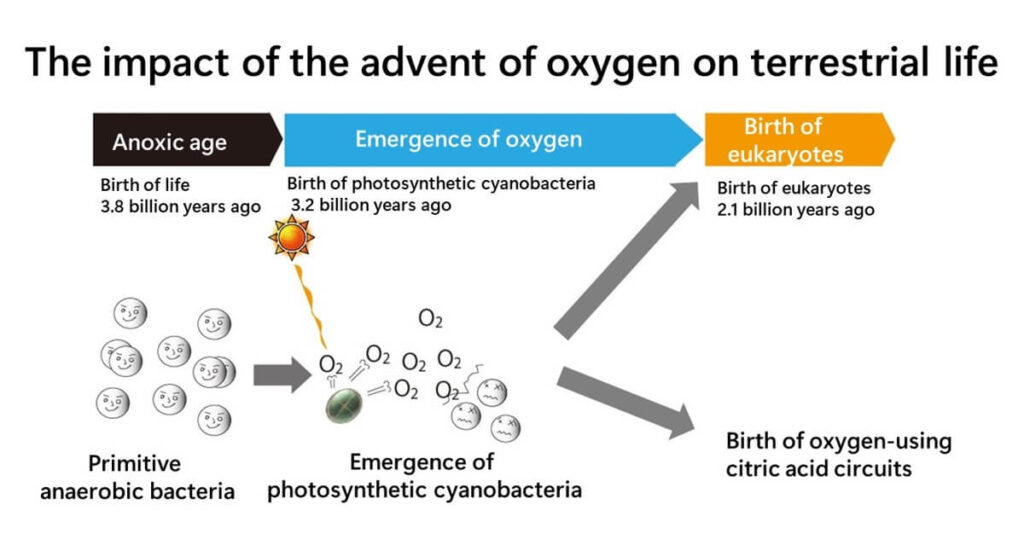
Oxygen’s toxicity stems from its ability to generate reactive oxygen species (ROS), which can damage cellular components like DNA, proteins, and membranes. To survive in this new oxygen-rich environment, organisms evolved various protective mechanisms, with catalase becoming one of the most critical defenses. By decomposing hydrogen peroxide, catalase prevents cellular damage and ensures the survival of organisms living in oxygenated conditions.
Real-World Example: Catalase Test in Quality Control
Let’s consider a practical example to highlight the role of the Catalase Test in quality control. Imagine a scenario where a customer contacts a food manufacturer, reporting that they found a cockroach in a can of food. This is a potentially serious issue, posing risks of a product recall and significant harm to the company’s reputation.

To investigate the claim, the quality control team performs a Catalase Test on the cockroach by applying hydrogen peroxide to it. If bubbles form, this indicates the presence of catalase, meaning the cockroach had been exposed to oxygen and was likely alive. This evidence suggests that the cockroach entered the can after it was opened, rather than being sealed inside during production.

This straightforward yet effective test allows the quality control team to determine whether contamination occurred during the manufacturing process or after the product left the factory. By providing clear, actionable insights, the Catalase Test plays a crucial role in resolving customer complaints and preventing similar incidents in the future.
Why Lactic Acid Bacteria Don’t Produce Bubbles in the Catalase Test
Why don’t lactic acid bacteria produce bubbles when subjected to the Catalase Test? While these bacteria also need protection from the harmful effects of oxygen, they achieve this through a different mechanism involving an enzyme called NADH peroxidase, rather than catalase. Unlike catalase, NADH peroxidase breaks down hydrogen peroxide into water without releasing oxygen as a by-product. Instead, it utilizes NADH, a coenzyme, to facilitate this reaction.
This unique enzymatic adaptation allows lactic acid bacteria to survive and thrive in oxygen-rich environments, despite their lack of catalase. Consequently, the absence of bubbles during the Catalase Test becomes a distinctive hallmark of lactic acid bacteria. This simple observation enables microbiologists to differentiate them from other Gram-positive bacteria that do produce bubbles due to the presence of catalase.
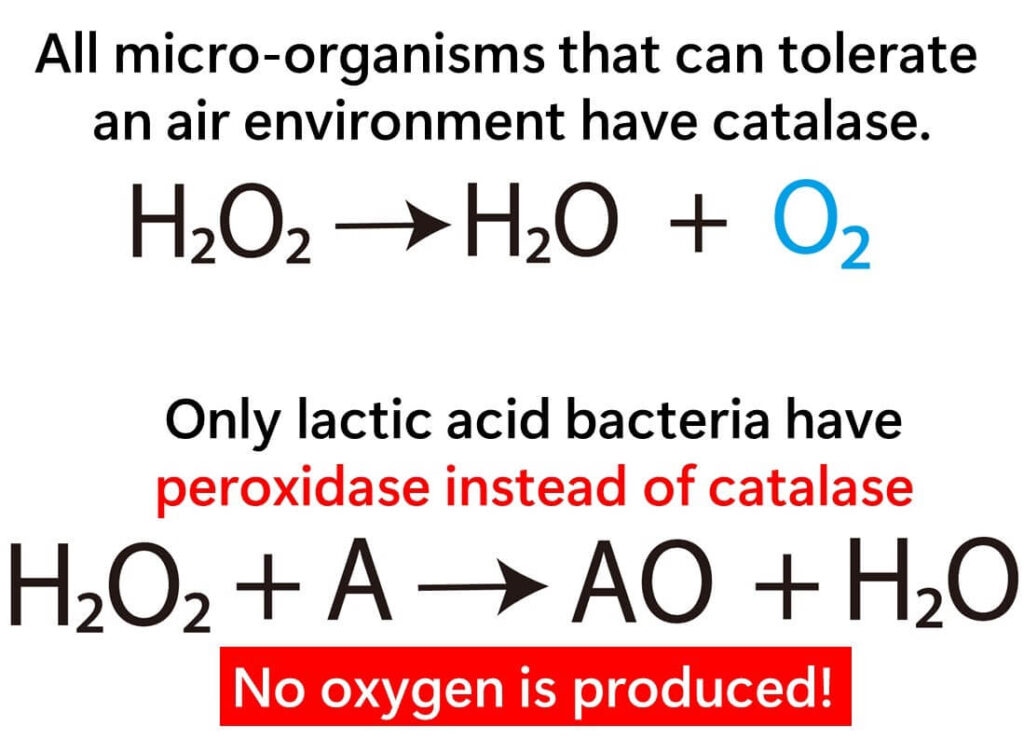
In summary, the reliance on NADH peroxidase instead of catalase ensures that no oxygen is released during the test, resulting in the absence of bubbles. This characteristic underscores the Catalase Test's importance as a practical and reliable tool for identifying lactic acid bacteria in microbiological studies.