Many companies are adopting the enzyme substrate medium method for their in-house testing of E. coli. But what about selecting the right medium or choosing the cultivation temperature? This article delves into the methods for testing E. coli based on the internationally recognized ISO standard, providing a detailed explanation.

ISO 16649 (Horizontal Method for Enumeration of β-Glucuronidase-Positive Escherichia coli)
ISO 16649, titled "Horizontal Method for the Enumeration of β-Glucuronidase-Positive Escherichia coli," is composed of three distinct methodologies. Users can choose any of these methods for application.
Note: The term "horizontal method" in ISO and other international standardizations refers to techniques that are applicable across a variety of samples and environments, not limited to any specific type or condition. In contrast, methods specific to certain samples or environments are termed "vertical methods."
- ISO 16649-1: This method uses a membrane and enzyme substrate (β-D-glucuronidase) medium for colony counting at 44 °C, specifically designed for detecting extremely damaged bacteria.
- ISO 16649-2: This involves the use of an enzyme substrate (β-D-glucuronidase) medium for colony counting at 44 °C.
- ISO 16649-3: This method utilizes an enzyme substrate (β-D-glucuronidase) medium for detection and employs the Most Probable Number (MPN) technique.
Among these, ISO 16649-1 aims to recover extremely damaged bacteria using a recovery medium on a membrane, and ISO 16649-3 refers to the MPN method which uses multiple liquid media. From a simplicity standpoint, food companies performing routine self-tests might not use ISO 16649-1 and ISO 16649-3 as frequently as ISO 16649-2. This article focuses on ISO 16649-2, discussing its practicality and its likely application by quality managers in the food industry.

ISO 16649-2: The Enzyme Substrate Medium Method for Colony Counting at 44°C
Let's discuss the steps of the ISO 16649-2:2001 method, updated in 2023, for the enzyme substrate medium method for colony counting at 44°C.
The steps of the ISO 16649-2:2001 method
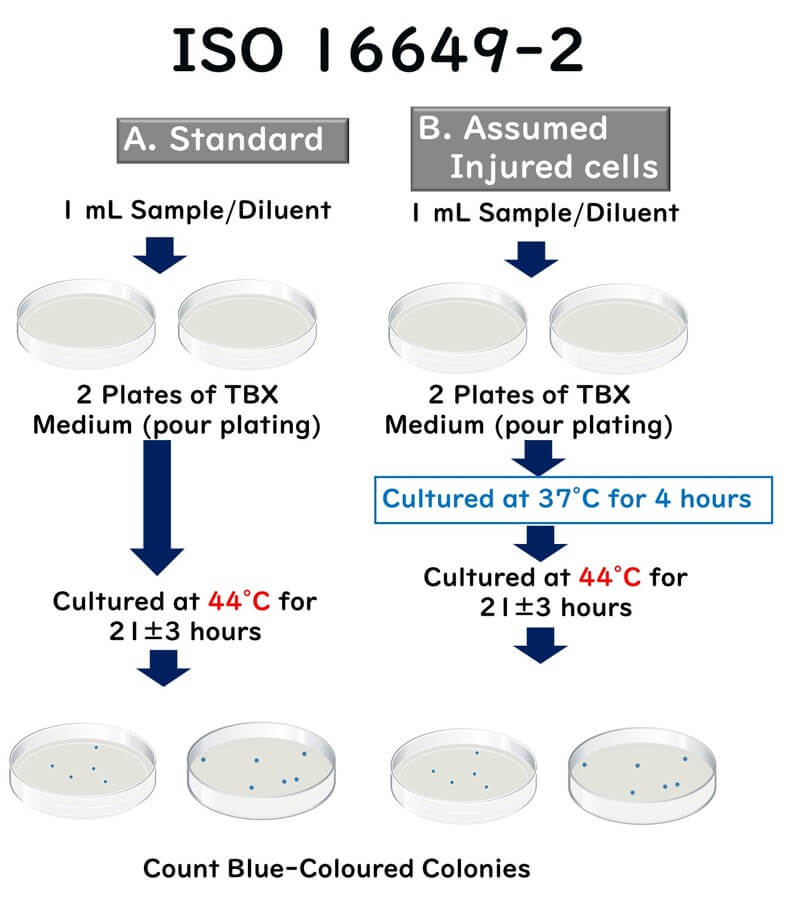
- Sample Transfer: Transfer 1 mL of the test sample (liquid) or 1 mL of the initial dilution solution (10-fold dilution) to a sterilized Petri dish.
- Inoculation: Inoculate two plates per dilution (further 10-fold dilutions can be done with a new sterile pipette as needed).
- Preparation of the Medium: Cool the tryptone bile xanthogenate (TBX) medium to between 44°C and 47°C in a water bath (ingredients detailed below). Pour approximately 15 mL of this medium into each Petri dish within 15 minutes after distributing the inoculum to ensure proper mixing.
- Setting the Plates: Place the inoculated dishes in a cool, flat area and carefully mix the sample liquid with the medium to solidify.
- Incubation: Invert the inoculated dishes so the bottom is up, and incubate at a controlled temperature of 44°C for 21 ± 3 hours.
- Optional Pre-Incubation for Damaged Cells: If the presence of damaged cells is suspected, first incubate at 37°C for 4 hours, then raise the temperature to 44°C and continue incubation for 21 ± 3 hours.
- Counting Colonies: After incubation, count the typical CFU of β-glucuronidase-positive E. coli when the typical CFU is less than or equal to 150 and the total (typical plus atypical) CFU count is less than or equal to 300 per plate.
Note on Damaged Cells: In food microbiology, damaged cells refer to microbes that have survived physical or chemical damage, such as heat, pH, water activity, or preservatives. These treatments increase the likelihood of microbes being present as damaged cells in food, which might still be viable but risk being deemed dead when cultured directly on selective media.
Quantitative Techniques: ISO 16649-2 also describes quantitative methods for calculating E. coli counts per gram of sample based on the colony numbers. This article focuses on detection, omitting detailed quantitative methods.
Understanding Medium Components
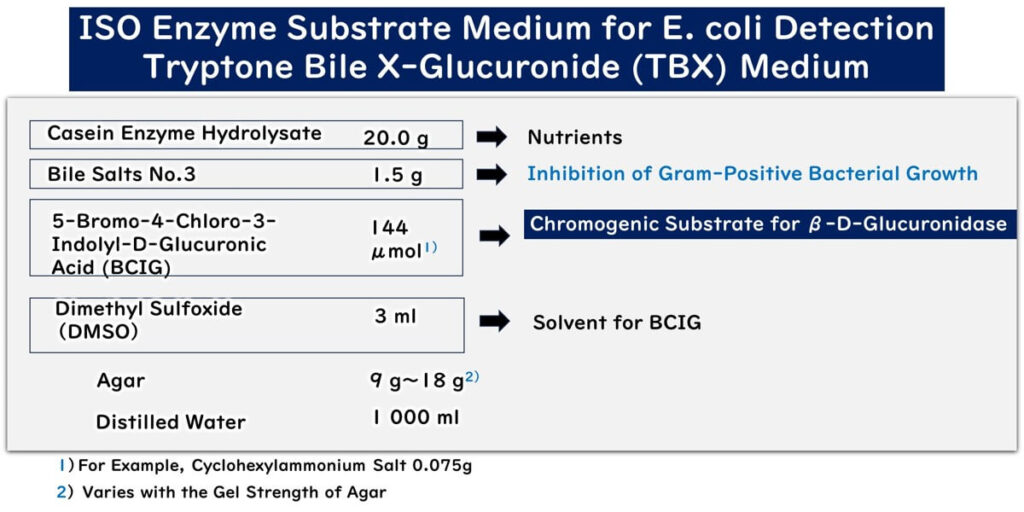
The components of Tryptone Bile Xanthogenate (TBX) medium are quite straightforward and are outlined as follows:
Nutrition
The medium contains casein enzyme hydrolysate as its sole nutrient source. This provides the necessary nutrients for bacterial growth, particularly supporting the proliferation of coliforms that can utilize this rich protein source.
Selectivity
Bile salts are included in the medium to inhibit the growth of Gram-positive bacteria, effectively allowing only Gram-negative bacteria to form colonies.
For a clear explanation on why bile salts inhibit Gram-positive bacteria, please see the referenced article.
Deciphering Culture Medium Components: A Key to Effective Bacterial Testing
Identification Principle
The chromogenic substrate 5-bromo-4-chloro-3-indolyl-β-D-glucuronide (X-glucuronide) is added to the medium. This substrate is utilized by Gram-negative bacteria possessing the enzyme β-glucuronidase, such as E. coli, which break down the substrate resulting in the formation of blue/green colonies.
For a more detailed explanation on how the enzyme substrate medium identifies E. coli, refer to the separate article in this blog
Are We Missing Something? A Closer Look at Substrate Enzyme Media as Indicators in Microbial Testing
To simplify, the identification of E. coli is based on detecting the enzyme β-glucuronidase, which is unique to this bacterium. The medium includes the substrate X-glucuronide, and the breakdown of this substrate results in the coloring that specifically marks the presence of E. coli.
Cultivation Temperature and Duration
Cultivate at 44°C ± 1°C for 18 to 24 hours, ensuring that the temperature does not exceed 45°C. The reason for setting the temperature at 44°C is the same as for the fecal coliform group; most Gram-negative bacteria, including but not limited to E. coli, cannot proliferate at this elevated temperature.
For an accessible explanation of what constitutes the fecal coliform group, please refer to the below blog article
However, if there is a possibility of the presence of damaged bacteria, immediate cultivation at 44°C might inhibit the growth of E. coli as well. Therefore, it's advisable to first allow for the recovery of damaged cells by incubating at a non-selective temperature of 37°C for 4 hours before shifting to the 44°C cultivation. This step is crucial to ensure the viability of potentially damaged E. coli, enabling accurate detection and enumeration.

UK Official Methods: Assumption of Damaged Bacteria
In the ISO 16649-2, there is an option for a detection protocol that considers damaged bacteria, making it the most straightforward and practical choice for routine testing in food companies.
Notably, in the UK, only the protocol for damaged bacteria from ISO 16649-2 is set as the official method for E. coli testing. Since it can be challenging for businesses to assess whether food contains stressed bacteria, the UK assumes all cells are stressed. Therefore, they have established a standard method using the stress pre-cultivation approach of ISO 16649-2 for all food and environmental samples.
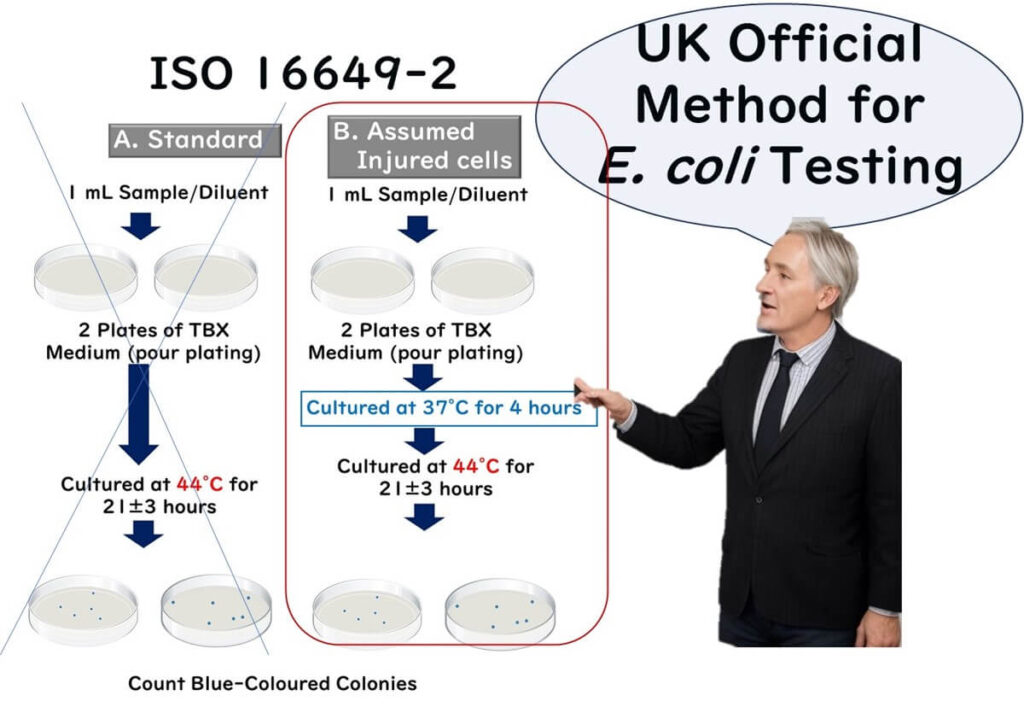
According to the UK's official method, E. coli is identified solely through the presence of blue colonies on TBX agar at 44°C, which indicate a positive β-glucuronidase reaction, thus eliminating the need for confirmatory tests.
From the perspective of this blog's author, the UK's approach is valued for its simplicity and practicality, as it removes the need for case-by-case judgement calls.
Conclusion
This article has focused on the ISO standard methods for E. coli testing adopted in the EU, particularly on the dilution method for colony counting (ISO 16649-2).
The defining feature of the ISO method is the cultivation temperature set at 44°C. This temperature is specifically chosen to suppress the growth of other coliform groups and Gram-negative bacteria, enhancing the selective cultivation of E. coli. The components of the TBX medium used primarily include bile salts, which allow the growth of all Gram-negative bacteria but do not contain specific selective agents that exclusively promote the growth of E. coli. This approach is evident in the ISO method's strategy to slightly elevate the cultivation temperature to selectively promote E. coli growth.
Conversely, some commercially available enzyme substrate media are set for cultivation at 37°C. This is designed to facilitate simultaneous differentiation between E. coli and other bacteria, as many from the coliform group struggle to proliferate at 44°C. However, if international certification bodies like the AOAC have proven the equivalence of these commercial media at 37°C to the ISO method at 44°C for detecting E. coli, then there should be no issues.
Understanding the ISO method is crucial when using enzyme substrate media to confirm the presence of E. coli. By comprehending the ISO standards, the interpretation and application range of the results can be significantly expanded.