In this article, we explore how pH impacts microbial growth and decay, focusing on the antimicrobial mechanisms of sodium acetate, propionic acid, and sorbic acid. We also examine the critical role of stomach acid in eliminating foodborne pathogens and its germicidal effects. Furthermore, we delve into how variations in acid resistance among pathogens influence the minimum infectious dose required to cause illness. Notably, most foodborne bacteria are highly sensitive to acidic environments, making pH control a vital tool in food safety management.
How pH Affects Microbial Growth Contro
When it comes to the relationship between microbial growth and pH, the response varies depending on the microorganism. However, as a general rule, maintaining a pH below 5.0 is crucial for controlling microbial growth in food.
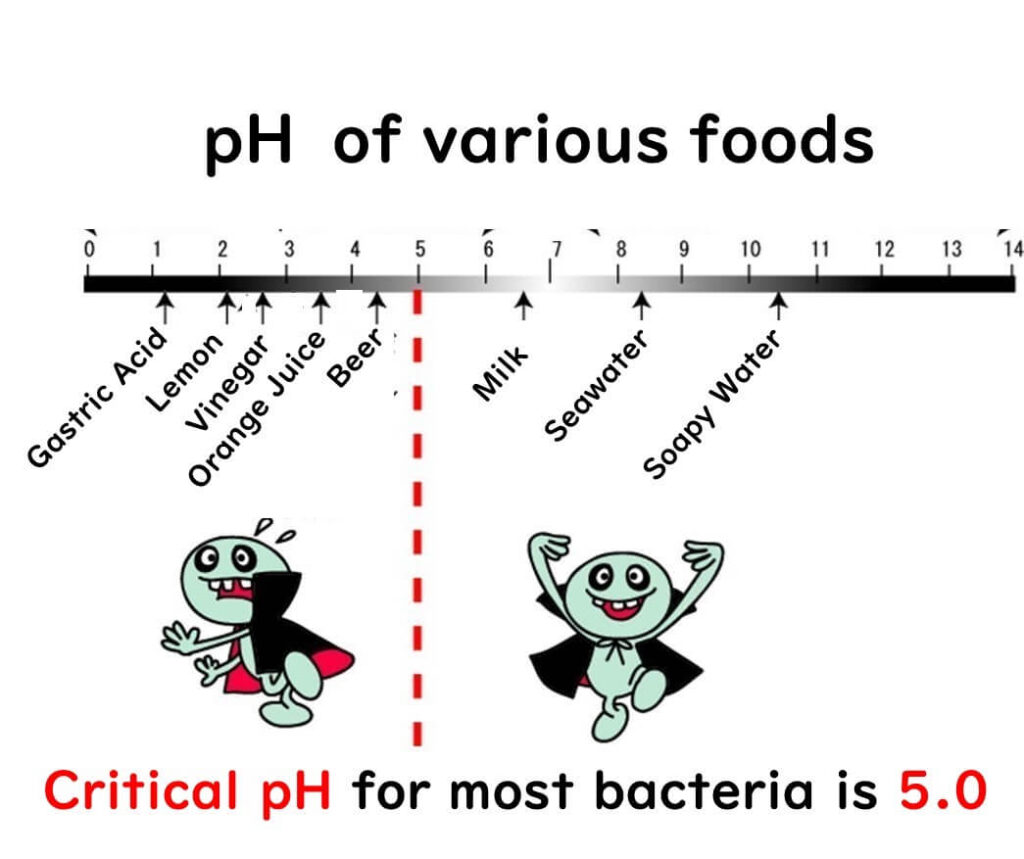
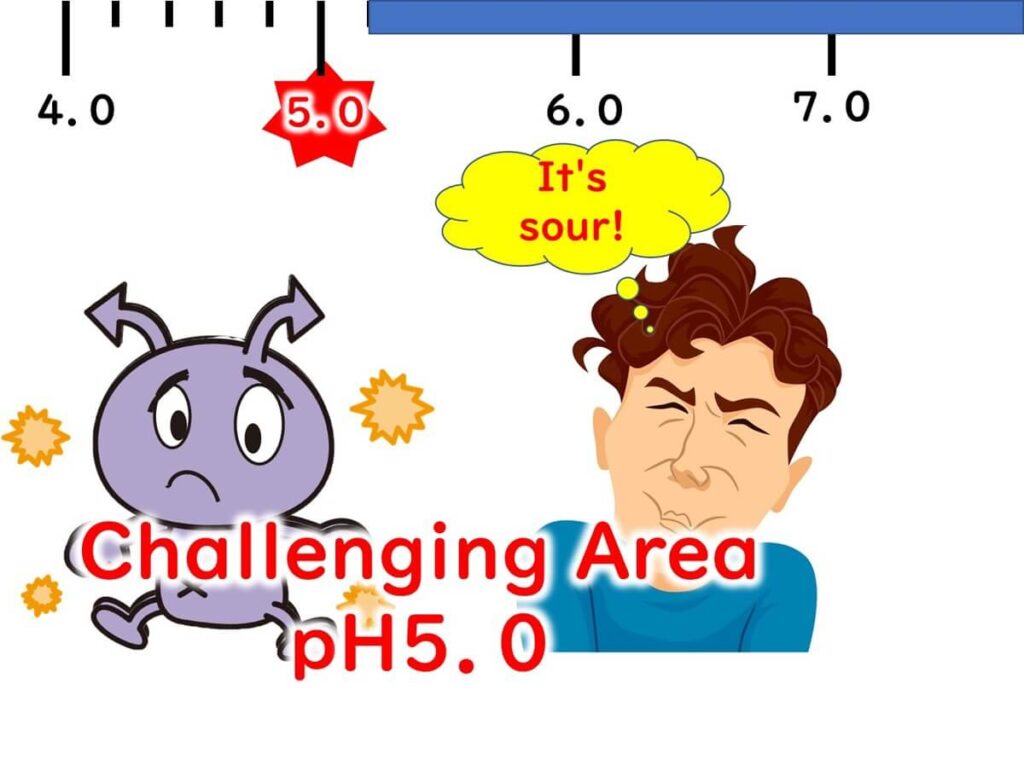
Microbial growth is generally not effectively inhibited if the pH is above 6.0. Based on my experience in growth control experiments with food companies, lowering the pH to around 6.0 typically has minimal impact. Significant growth suppression begins only when the pH drops below 6.0. Even then, at pH levels between 5.0 and 6.0, many microbes can still grow, albeit more slowly. However, once the pH falls below 5.0, the proliferation of most microbes ceases.
For effective microbial control in food products, targeting a pH below 5.0 is essential. Foods like beer, soy sauce, juices, and carbonated drinks are classic examples where this principle is applied.
That said, maintaining a pH below 5.0 introduces acidity, which may affect the product's sensory qualities—a challenge for food product developers. Additionally, it’s important to note that the minimum growth pH varies between microorganisms. For instance, while lactic acid bacteria can thrive at pH levels below 4.0, most foodborne pathogens have minimum growth pH levels between 4.0 and 5.0.
A practical example is Salmonella, which has a minimum growth pH of 3.7. This figure does not indicate active growth at that level but rather the point where some cells may divide. In practical food quality control, maintaining a pH below 5.0 is typically sufficient to prevent significant growth of foodborne pathogens.
The following table, published by the US FDA, provides the minimum growth pH for several common foodborne pathogens.

How Organic Acids Control Microbial Growth
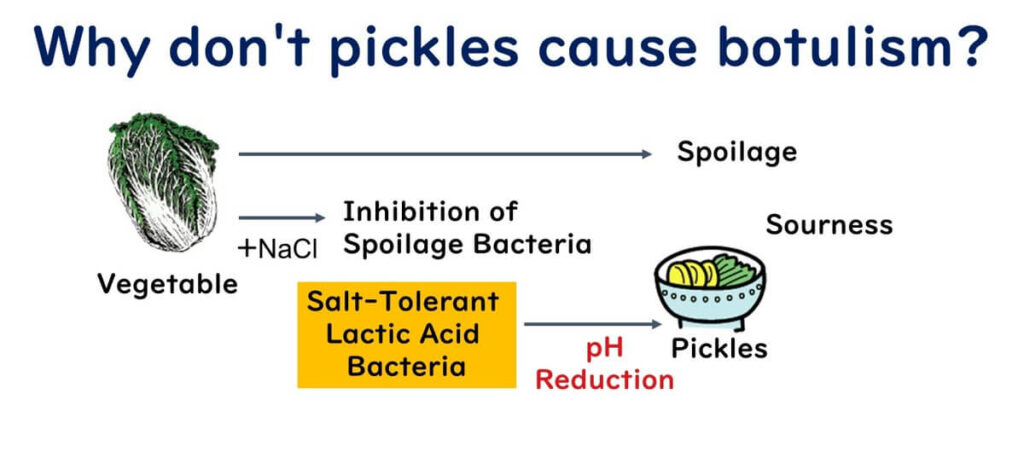
Controlling microbial growth in food often involves the use of organic acids. Commonly used organic acids include acetic acid, lactic acid, sorbic acid, propionic acid, and benzoic acid. For example, vegetables left at room temperature can spoil due to spoilage bacteria like Pseudomonas that adhere to their surfaces. However, in pickled vegetables, these spoilage bacteria are unable to proliferate because of the low water activity—a concept that will be explained in a future article.
In contrast, salt-tolerant lactic acid bacteria naturally present on the surfaces of vegetables can thrive in such conditions. These bacteria ferment the vegetables, producing lactic acid and reducing the pH of the pickles. This reduction in pH gives pickles their characteristic sour flavor. Importantly, when the pH drops below 4.6, even Clostridium botulinum spores, if present, cannot grow.
Similarly, most spoilage bacteria cannot survive in such low-pH environments. This makes lactic acid, one of the organic acids, particularly effective at suppressing the growth of foodborne pathogens and spoilage bacteria in pickled vegetables. The same antimicrobial mechanism of organic acids has been adapted for use in other foods as preservatives, making them a key tool in food safety and quality control.
The Mechanism Behind Organic Acids as Preservatives
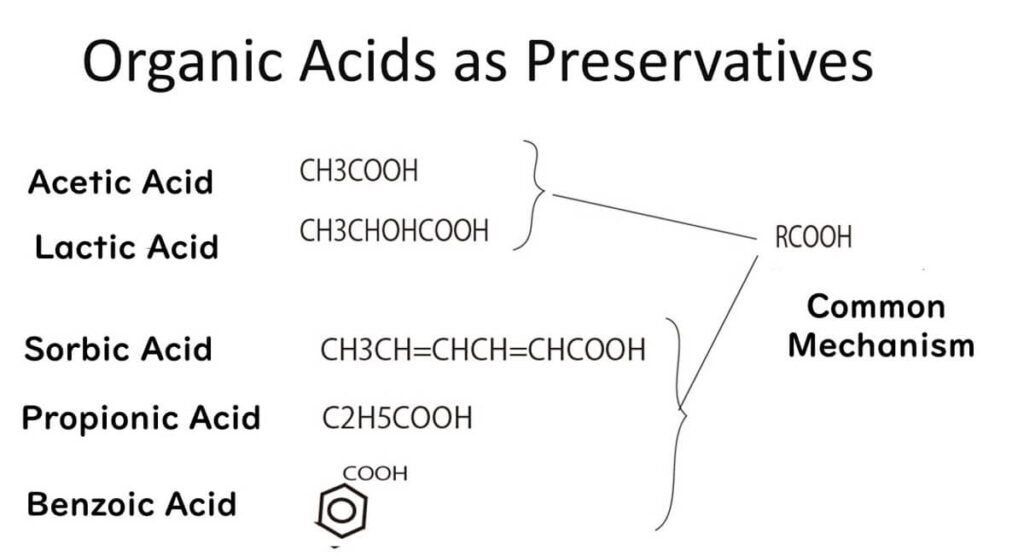
Organic acids play a crucial role as preservatives in food by inhibiting microbial growth. Their antimicrobial action can be understood through their chemical structure and interaction with microbial cells.
A common feature of organic acids is the presence of a carboxyl group, which is hydrophilic. Attached to this carboxyl group are various hydrophobic functional groups. For instance, acetic acid contains a simple methyl group, while propionic acid has a two-carbon chain, and benzoic acid includes a benzene ring. While the specifics of each hydrophobic group differ, the key principle is that all organic acids share hydrophobic functional groups essential for their antimicrobial activity.
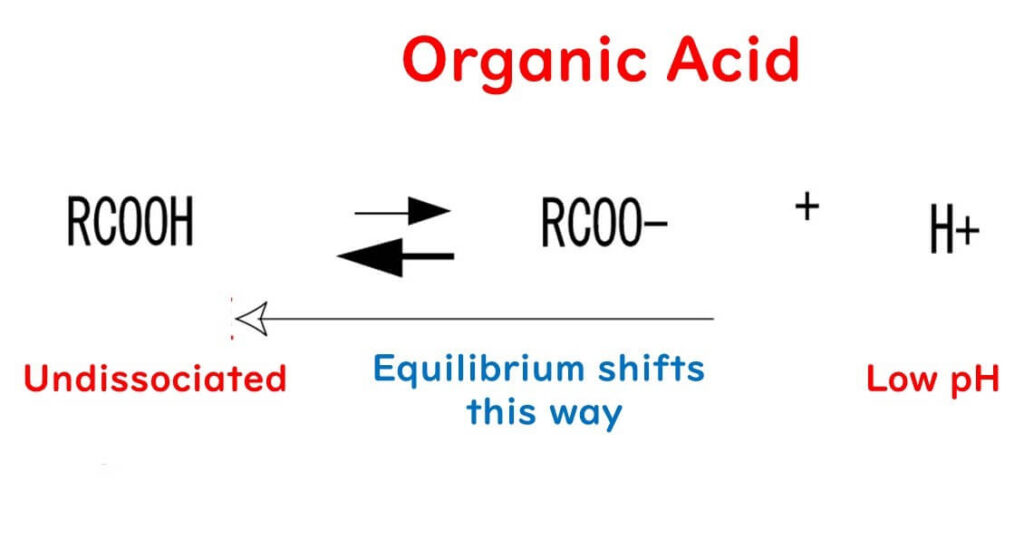
How do organic acids inhibit microbial growth?
For organic acids to be effective, they must lower the food's pH to sufficiently acidic levels. In acidic environments, organic acids like acetic acid exist primarily in their non-dissociated form (R-COOH), which is more hydrophobic due to the influence of the R group.
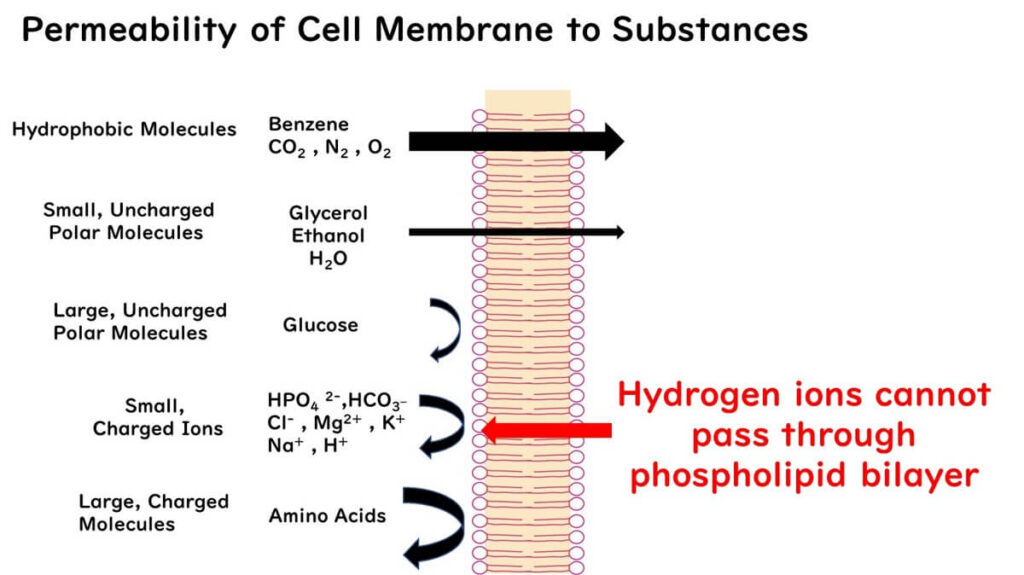
To understand their action, it’s necessary to consider the properties of microbial cell membranes. Cell membranes are composed of a phospholipid bilayer that allows hydrophobic compounds to pass through easily but restricts the movement of hydrophilic substances, including charged ions like hydrogen ions (H+).
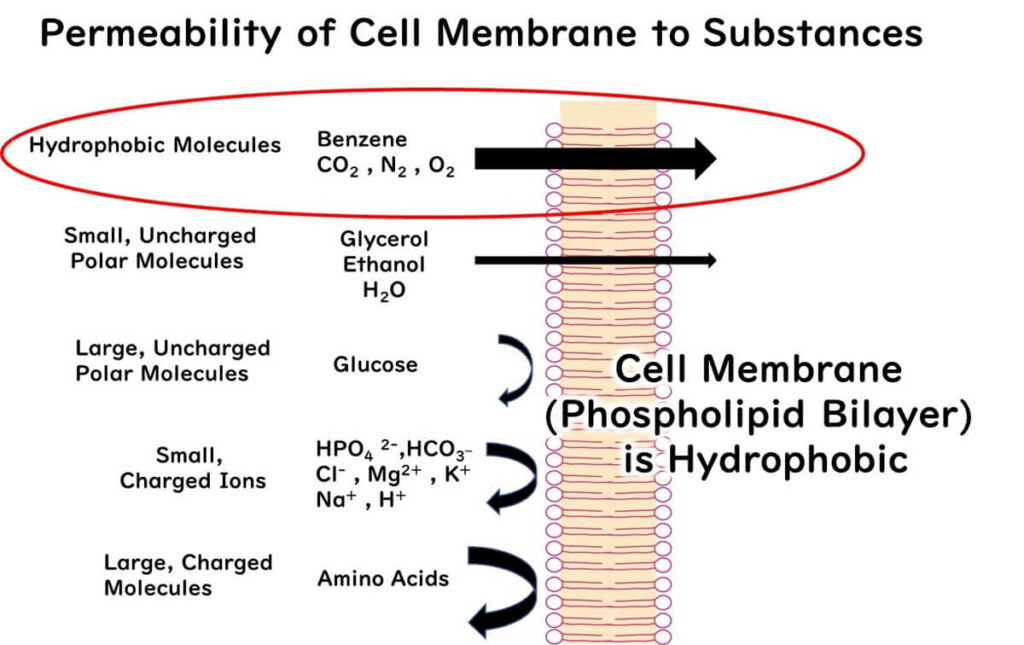
This characteristic allows non-dissociated organic acids to infiltrate microbial cells efficiently.
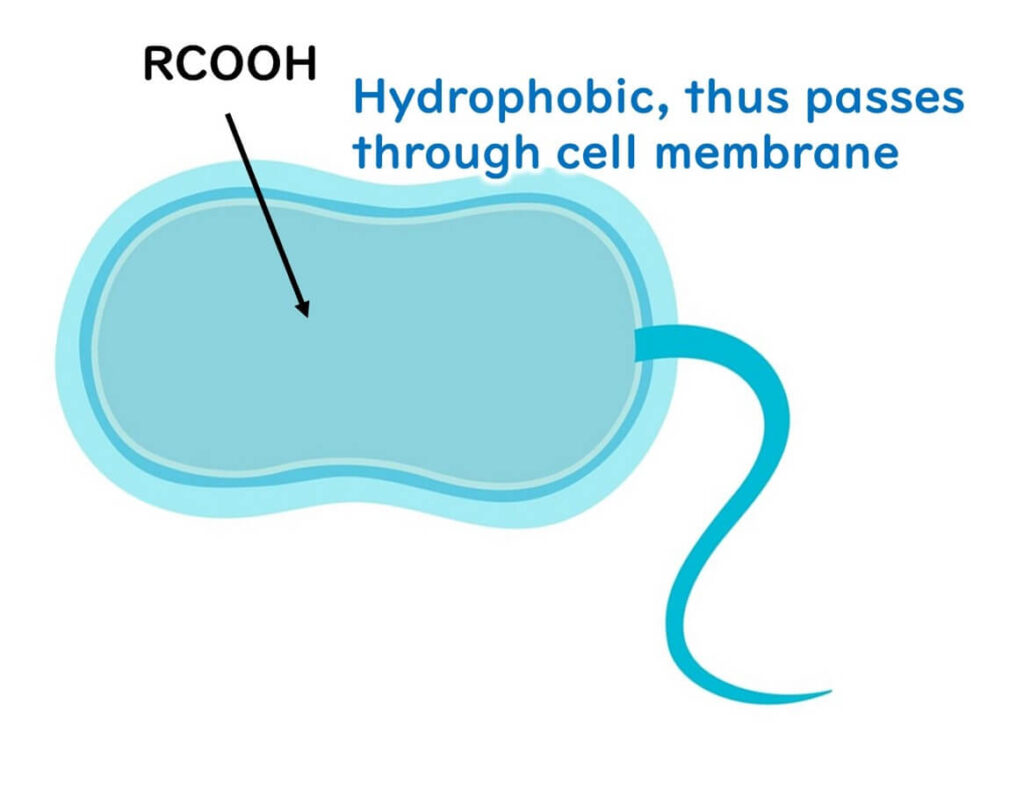
Once inside the cell, where the internal pH is close to neutral, organic acids dissociate into RCOO- and H+.
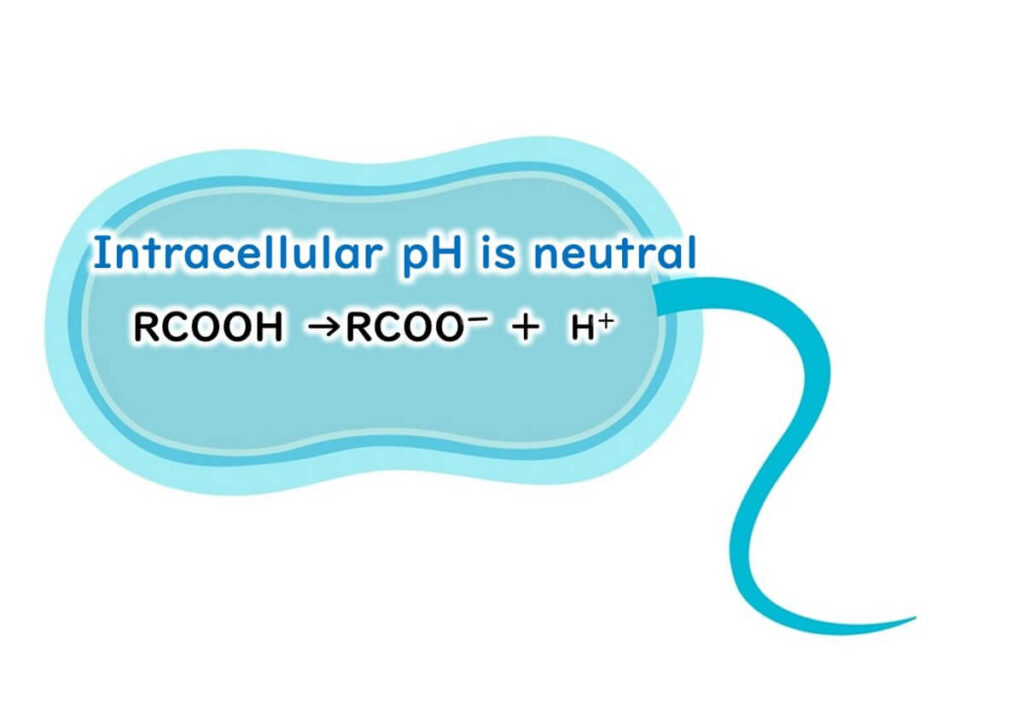
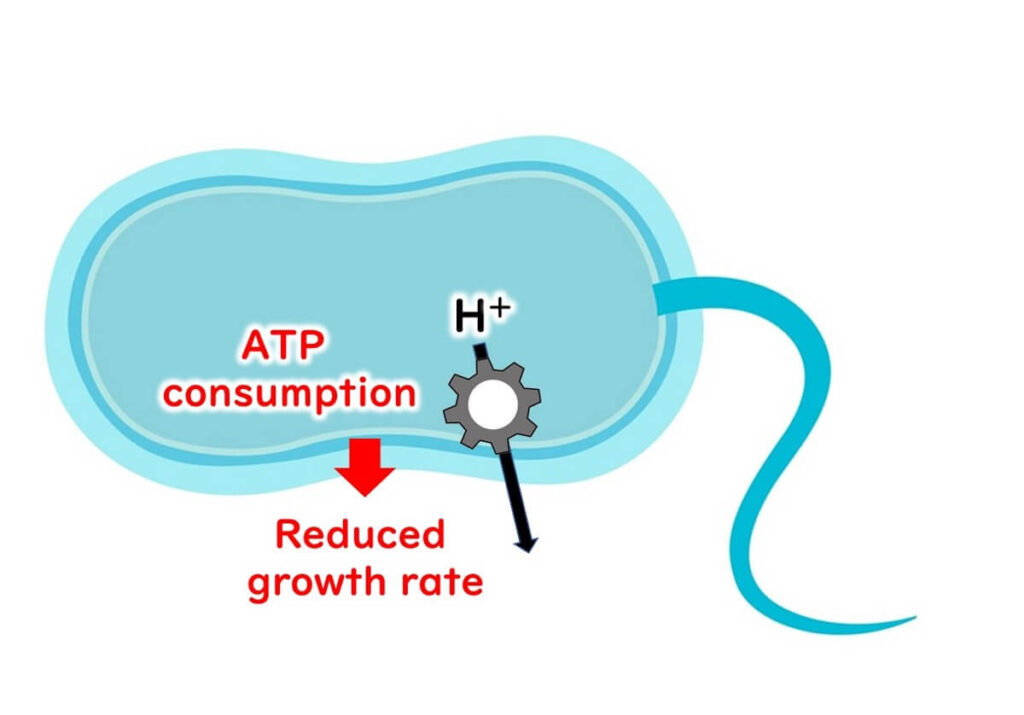
This dissociation leads to an accumulation of hydrogen ions [H+] inside the cell. To maintain internal stability, the microbe must actively expel these hydrogen ions using membrane proteins, a process that consumes significant amounts of ATP—the primary energy source for cellular functions.
The depletion of ATP disrupts microbial energy metabolism, ultimately slowing down or halting proliferation. This mechanism of energy exhaustion explains how organic acids inhibit microbial growth, regardless of the specific structures of their hydrophobic functional groups. These shared principles underline the effectiveness of organic acids as preservatives.
The Germicidal Role of Stomach Acid
The stomach plays a crucial role in protecting the body from foodborne pathogens through its production of gastric acid, primarily composed of hydrochloric acid. This acid lowers the stomach’s pH to below 3.0, creating a highly acidic environment that effectively kills most microbes. In this way, stomach acid acts as a natural sterilizer, eliminating harmful bacteria that enter the body with food.
While the stomach is commonly associated with digestion, its germicidal function is equally important. Although the stomach physically softens food to aid digestion, this process can still occur without it. For example, individuals who have undergone a total gastrectomy due to stomach cancer can still digest food because digestive enzymes are secreted in the small intestine. However, without a stomach, the body loses its ability to kill foodborne microbes with gastric acid, making these individuals more vulnerable to infection-based food poisoning.
Even when consuming the same contaminated food, not everyone becomes infected. This variation in susceptibility depends not only on immune strength but also on the stomach's germicidal power. A healthy stomach with robust acid production significantly reduces the risk of foodborne infections by neutralizing pathogens before they can reach the intestines.

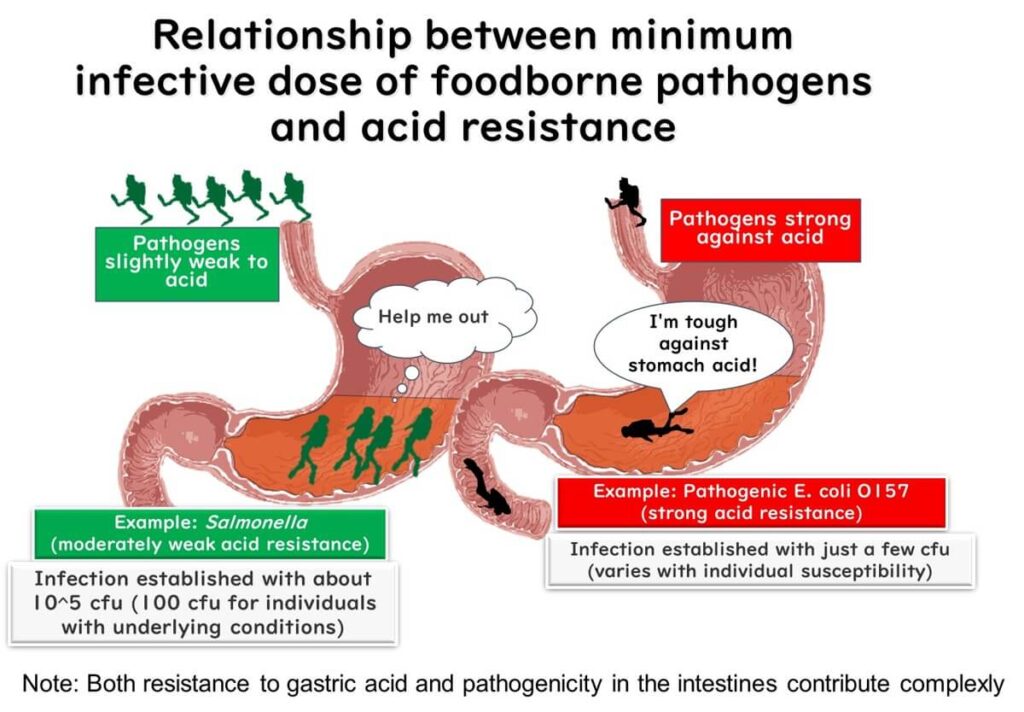
How Acid Resistance Impacts Foodborne Infections
The minimum infectious dose of foodborne pathogens is closely related to their resistance to acidic environments. Comparing two common pathogens, enterohemorrhagic Escherichia coli (EHEC) and Salmonella, illustrates this relationship.
Salmonella requires a higher number of bacteria to cause infection because it is relatively weak against stomach acid. When large quantities of Salmonella cells enter the stomach, the majority are neutralized by gastric acid. Only a small fraction survive to reach the intestines, meaning that a higher initial number of bacteria is necessary to cause illness.
In contrast, enterohemorrhagic Escherichia coli (EHEC) is more resistant to acidic conditions. Even a small number of EHEC cells can survive the stomach's acidic environment and reach the intestines. This enhanced acid resistance means that EHEC can cause infection with a much smaller initial dose compared to Salmonella.
Understanding the variation in acid resistance among pathogens is critical for food safety management. It highlights the importance of controlling bacterial contamination levels, particularly for pathogens like EHEC that can infect with minimal exposure.