While HACCP aims to reduce reliance on end-product testing, microbial testing still plays a vital role in effective hygiene management. This article explains when microbial testing is still necessary, how it supports HACCP implementation, and why it should not be entirely replaced by monitoring alone—even in highly controlled environments.
Introduction
Will the introduction of HACCP eliminate the need for microbial testing?
Of course not.
So, what kind of microbial testing remains necessary?
This section outlines the essential role of microbial testing in hygiene management under HACCP.
1. Planning HACCP Requires Deep Knowledge of Microbiology
Advanced Food Microbiology is Essential for Hazard Analysis and CCP Setting
To conduct Hazard Analysis (HA) and establish Critical Control Points (CCPs), a solid understanding of food microbiology is crucial.
Mistakes in HA and CCP design can lead to food poisoning instead of preventing it.
You must thoroughly understand how your product’s characteristics relate to microbial hazards.
Identifying Microbiological Hazards
This requires in-depth and practical microbiological knowledge to:
- Identify hazards across all production stages—from raw materials to processing, environment, and distribution
- Understand which microbial hazards are specific to each stage

Establishing CCPs Based on Scientific Evidence
Once hazards are identified, CCPs must be defined. This requires detailed consideration of your product’s characteristics—such as raw material contamination levels, water activity, and pH.
CCPs cannot be determined from general information alone.
It is advisable to review scientific literature and synthesize insights specific to your products.

When Literature Is Not Enough
In many cases, scientific papers offer enough evidence to set CCPs. However, unique product conditions may demand additional microbial experiments.
In such cases, accurate microbiological testing capabilities are essential.
2. Microbial Testing in the Operational Phase
Is Microbial Testing Still Necessary After HACCP is Implemented?
Yes. Microbial testing remains vital in various scenarios during actual food production.
2.1 Compliance with Microbial Standards
Food sanitation laws define microbial standards by product type.
Microbial testing is needed to confirm compliance, as required by regulations.
2.2 Testing of Raw Materials
Raw materials entering a factory are effectively a black box.
If microbial contamination in raw materials is high, CCPs may not function properly.
Therefore, in addition to trusting supplier audits:
- Periodic microbial testing of raw materials is needed
- Testing establishes baseline trends and supports risk assessment

2.3 Why Not Eliminate All Microbial Testing?
HACCP Aims to Replace Testing with Monitoring
Ideally, microbial risk is controlled through monitoring of sterilization temperature and time at CCPs—not through microbial testing.
But not all foods allow this ideal.
Below are examples of foods where microbial testing is:
- Almost unnecessary, and
- Still essential
3. When Microbial Testing is Unnecessary
Example: Canned Foods and Shelf-Stable Products
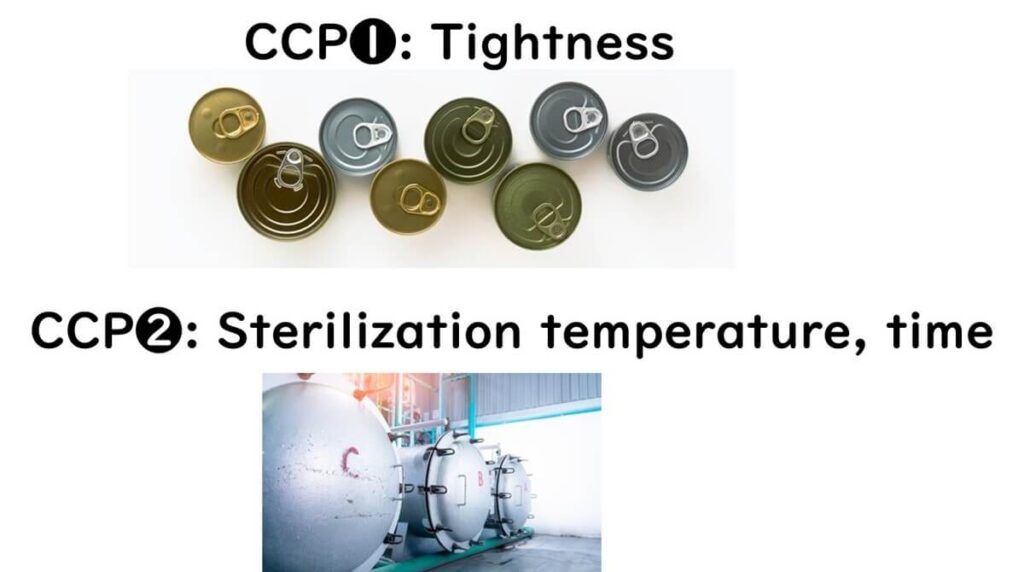
For canned foods sterilized under pressure:
- Microbial hazard = Clostridium botulinum
- Two clear CCPs:
- Achieve F₀ sterilization
- Ensure proper sealing

If both are controlled:
- Clostridium botulinum risk is virtually eliminated
- Microbial testing becomes unnecessary

NASA introduced HACCP starting with canned space food, showcasing this ideal application.
👉 For more details on retort sterilization:
Heat Sterilization of Food (Retort Sterilization)
4. When Microbial Testing Remains Essential
Example: Bento Boxes and Prepared Foods
Unlike canned foods, prepared dishes and bento boxes:
- Use diverse ingredients
- Often rely on pasteurization below 100°C
- Involve post-process handling, increasing contamination risk
CCPs are not straightforward.
Even with HACCP in place, microbial testing is still necessary.

4.1 Why Lot-Based Testing Isn’t Enough
Many companies conduct microbial testing only on final product lots.
However, testing just part of a lot does not guarantee total safety.

This undermines the entire concept of HACCP.
5. Microbial Testing to Verify CCP Functionality
Shift in Purpose: Not Just Lot Testing
So, what kind of testing is appropriate?
👉 Shift from shipment-time random testing
👉 To routine testing that verifies CCPs are functioning correctly
It’s like a car inspection:
- If every part functions, the car is safe
- But over time, parts may degrade

CCPs require regular inspection and adjustment, too.

5.1 When CCP Adjustments Are Needed
Adjustments may be required if:
- Raw material contamination levels exceed expectations
- New microbial species or strains emerge
5.2 The True Purpose of Microbial Testing
Microbial testing should:
- Confirm CCPs are functioning
- Be conducted regularly
- Not be relied upon to validate each lot’s safety
Even when testing is done:
- Good results ≠ Guaranteed safety
- They only confirm proper system functioning
5.3 Exception: Final Product Testing Can Be Useful
While final testing cannot guarantee safety, it allows for:
- Immediate intervention when abnormalities are found
This topic is thoroughly discussed in a paper by Dr. Zwietering, chair of ICMSF .
👉 For more details:
Relevance of microbial finished product testing in food safety management
6. Microbial Testing of the Factory Environment
Focus Should Shift to Environmental Testing
Under HACCP, hygiene management should prioritise:
- Factory environment testing
- Over final product testing
Many food poisoning cases arise from secondary contamination within the factory.

👉 Details on how to conduct environmental testing will be covered in an upcoming article.
Conclusion
To achieve effective hygiene management under HACCP:
- Combine CCP records with microbiological test data
- Relying on “validation documents” alone is insufficient
At the same time:
- Microbiological test data alone is also not enough
- Both management records and test data must work together
And most importantly:
Microbial testing of final product lots does not guarantee the safety of the lot.

