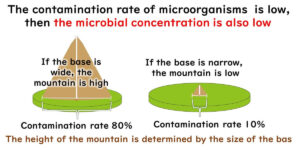
In the context of food factory hygiene management, the Environmental Monitoring Programme (EMP) plays a critical role as a tool for microbiological testing. Numerous cases of foodborne illness and product recalls have shown that the implementation of HACCP alone is not sufficient to prevent microbiological contamination. This article outlines the fundamental principles of environmental microbiological monitoring in food manufacturing plants.
Why is Environmental Monitoring Essential in Food Hygiene Management?
Despite the introduction of HACCP systems in food factories, many global foodborne illness cases stem from deficiencies in prerequisite programmes. These deficiencies typically relate to inadequate validation and verification of sanitation (including facility hygiene and design) and GMP (Good Manufacturing Practices, including hygiene zoning).
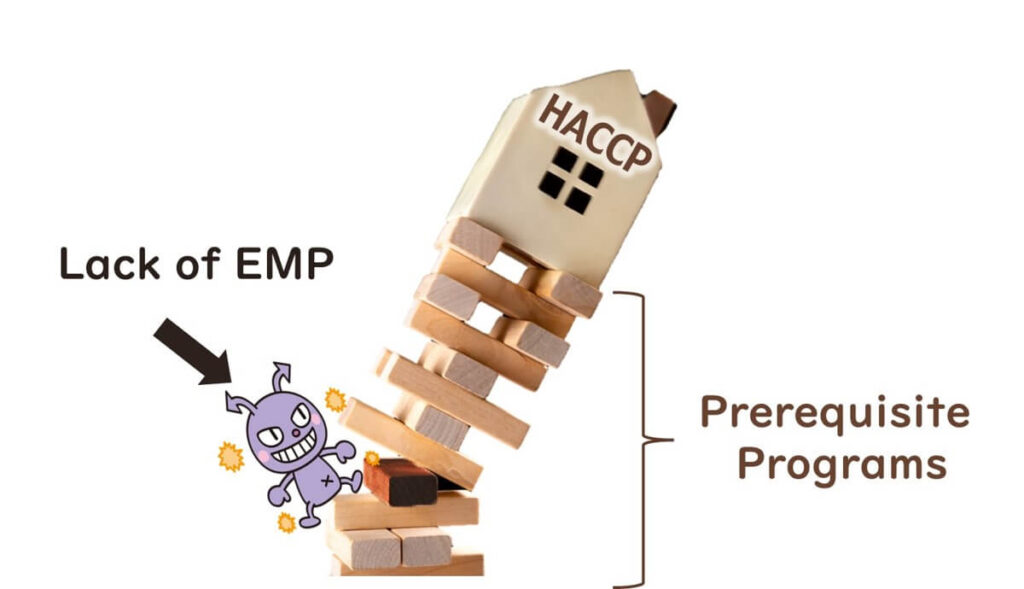
This is particularly evident in foods that are packaged after heat treatment (e.g., ready-to-eat products), where secondary microbial contamination frequently occurs.
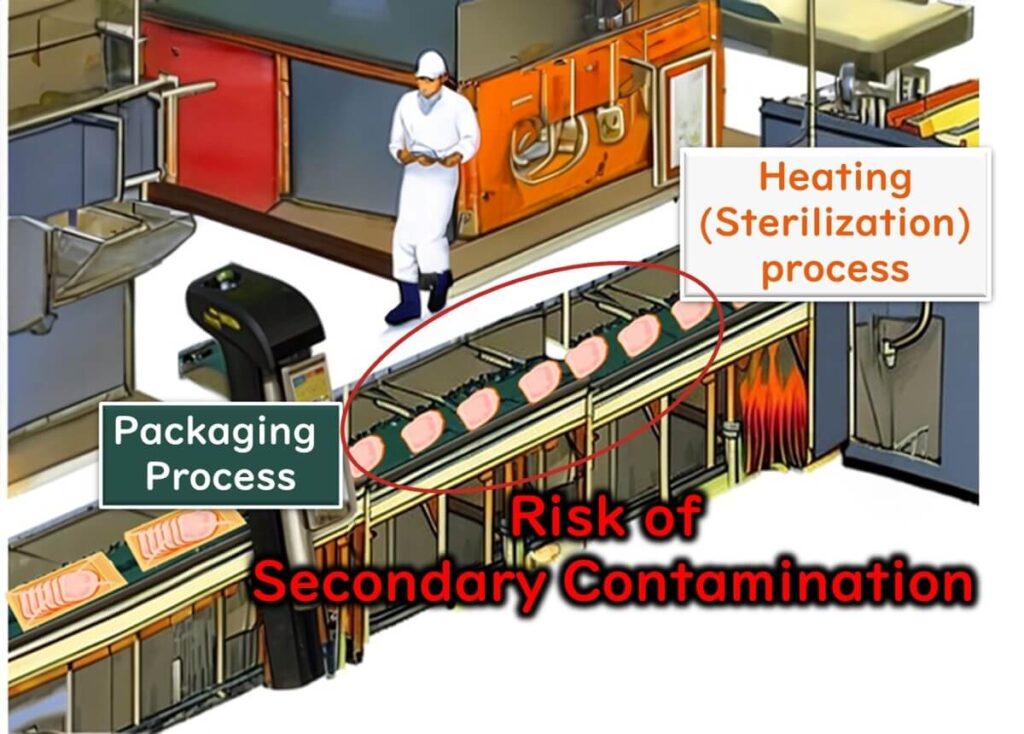
Therefore, as a prerequisite to HACCP, implementing an Environmental Monitoring Programme (EMP) is essential for effective microbiological testing and contamination control.
The Lack of a Standardised Protocol in Environmental Monitoring
Although several guidelines and standards have been published (e.g., USDA FSIS 2014, FDA 2017, ISO 18593:2018 、3M & Cornell University2019, EN 17141:2020), there is currently no single, internationally recognised protocol for environmental monitoring in food manufacturing.

Most documents focus on monitoring pathogenic microorganisms, particularly Listeria monocytogenes and Salmonella. However, few address indicator organisms such as E. coli, and even fewer consider spoilage microbes like Pseudomonas, lactic acid bacteria, moulds, and yeasts.
Monitoring protocols must be tailored to the characteristics of each factory, depending on factors such as:
- Type of food produced
- Facility layout and design
- Scale and automation of operations
- Number of employees
- Sterilisation processes used
Objectives of Environmental Microbiological Monitoring
The main aims of monitoring in a food factory setting include:
- Verifying the effectiveness of cleaning and sanitising procedures
- Detecting the presence of specific pathogens (persistent or transient)
- Identifying potential contamination sources
- Understanding the microbial ecology within the plant environment
Basics of Microbiological Environmental Monitoring
Each environmental monitoring programme should be custom-designed. The following outlines common practices based on international guidelines.
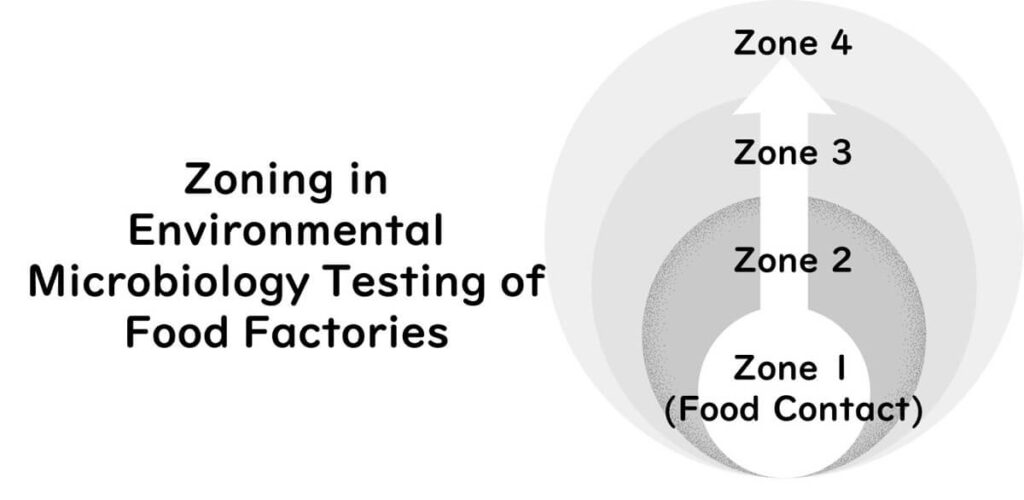
Zoning for Environmental Sampling
Zoning is a key component of environmental monitoring. Sampling areas are commonly divided into three or four zones:
- Zone 1: Exposed food contact surfaces (highest risk)
- Zone 2: Non-food contact surfaces adjacent to food zones
- Zone 3: More distant surfaces within or near the processing area
- Zone 4: Areas outside the processing environment
When using a three-zone model, Zones 2 and 3 are combined.
Zone 1 Examples
- Conveyor belts transporting unpackaged food
- Food handling tables
- Internal surfaces of pipes and mixing vessels
- Cooling rack covers
- Filler nozzles
- Storage box interiors
- Utensils for food handling

Zone 2 Examples
- Walls, ceilings, and floors near exposed food
- Equipment parts beyond food contact surfaces
- Areas within the same room as Zone 1

Zone 3 Examples
- Forklifts, hand trucks, and carts
- Walls, floors, and drains near Zone 2

Zone 4 Examples
- Locker rooms, toilets, and corridors
- Entrances, receiving areas, and warehouses
Preventive contamination control measures should be in place when moving from Zone 4 to Zone 3 and then to production zones.


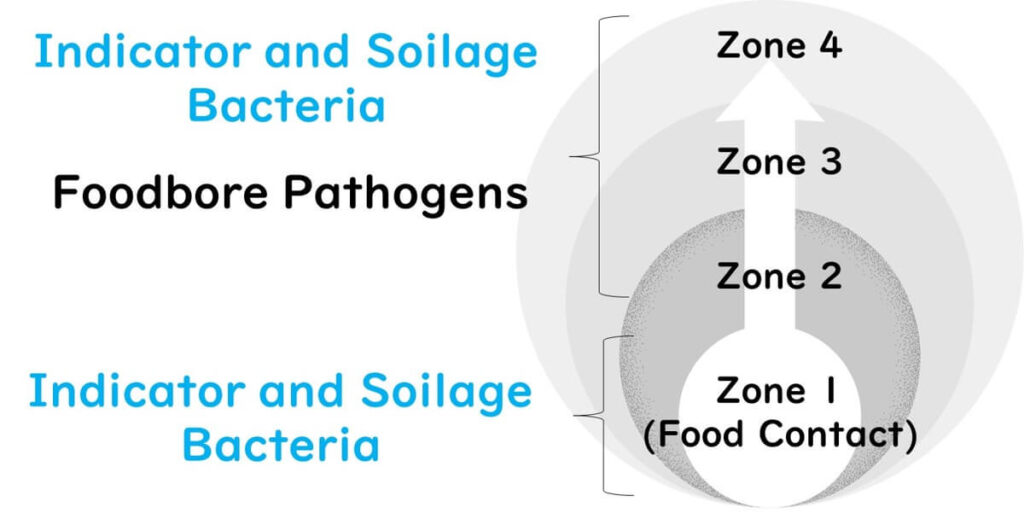
Target Microorganisms for Environmental Monitoring
The organisms targeted vary by zone and purpose:
- Zones 1–4: Indicator and spoilage bacteria
- Zones 2–4: Indicator bacteria, spoilage bacteria, and pathogens
Pathogens are typically excluded from routine Zone 1 monitoring due to the delay in product release caused by testing. Pathogen monitoring in Zone 1 is reserved for high-risk situations.
Frequently Targeted Pathogens
Listeria monocytogenes
- Key concern in ready-to-eat (RTE) foods
- Known to form persistent biofilms
Salmonella
- Can persist in factory environments
- Causes secondary contamination (e.g., in peanut butter, chocolate)
Cronobacter sakazakii
- Critical for powdered infant formula
- Capable of long-term survival in dry environments
Designing a Sampling Plan
Phase 1: Random Sampling
Initial sampling should be random to ensure all areas are fairly assessed. All food contact surfaces must be included to confirm post-startup hygiene effectiveness.

Phase 2: Combined Random and Targeted Sampling
Using Phase 1 data, Phase 2 incorporates both:
- Random sampling
- Targeted sampling based on prior results or environmental risk (e.g., drain backflow, condensation, sanitation concerns)
Example: For each production line, 3–5 samples might be taken—1–2 targeted, the rest random.
In areas with known hygiene problems, increase sampling frequency accordingly.

Sampling Frequency Guidelines
Annual and Monthly Planning
Sampling frequency depends on:
- Product type and volume
- Production frequency
- Facility age and design
- Shared use of equipment between raw and RTE foods
Example: FDA Guidance for Listeria monocytogenes (2017)
- No Listeria growth: Monthly sampling
- Detected growth: Weekly or more frequent sampling
- Positive Listeria spp. results: Increase sampling rate
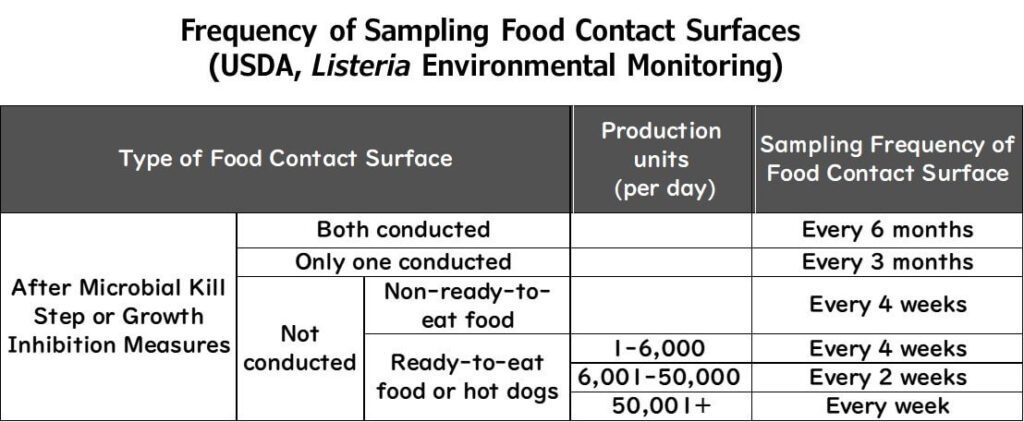
Example: USDA FSIS Recommendations
Sampling Zone 1 depends on whether sterilisation or growth suppression measures are in place.
Site Risk Classification
Sampling sites may be categorised as:
- High-risk: Always sampled
- Rotational: Sampled in turn
- Random: Sampled without a fixed plan
- Free-choice: Chosen by the sampler
Timing of Daily Sampling
Sampling can occur:
- Before production: To verify cleaning/sanitising
- During production: For general monitoring
- After production: To detect contamination over time

Recommended Practices
- Run equipment before sampling to dislodge hidden microbes
- ISO 18593:2018 suggests sampling at least two hours after production begins or at the end of a production cycle
- If contamination is suspected during production, sample while machinery is operating

Swab Testing Methods and Areas
ISO 18593:2018 recommends:
- 1,000–3,000 cm² swabbed for detecting specific pathogens
- 100 cm² swabbed for general microbial counts
USDA FSIS advises sampling the largest possible area, ideally 30 cm x 30 cm. If equipment is small (e.g., buttons), smaller areas are acceptable.

Recommended Reading and References
For those seeking further information, the following resources are recommended:
- Environmental monitoring program to support food microbiological safety and quality in food industries: A scoping review of the research and guidelines Food Control,130, 108283 (2021)
- Guidance for Environmental Testing for Listeria: USDA FSIS (2014)
- Draft Guidance for Industry: Control of Listeria monocytogenes in Ready-To-Eat Foods: FDA (2017)
- Microbiology of the Food Chain – Horizontal Methods for Surface Sampling: ISO 18593:2018 (2018)
- Environmental Monitoring Handbook for the Food and Beverage Industry: 3M & Cornell University (2019)
- Environmental Monitoring Program to Support Food Microbiological Safety and Quality in Food Industries: A Scoping Review of the Research and Guidelines: Food Control, 130, 108283 (2021)
Conclusion and Upcoming Case Studies
This article has outlined the foundational concepts of microbiological environmental monitoring in food factory hygiene management. However, theory alone is not sufficient—real-world case studies provide practical insights into applying these principles effectively.
In future blog posts, we will introduce individual monitoring case studies based on actual factory scenarios. These will be presented intermittently and aim to deepen practical understanding of microbiological monitoring for hygiene control.