In this article, I’ll be discussing a fascinating paper that examines the effectiveness of peracetic acid as a disinfectant in poultry processing plants, particularly during the chilling process of broilers. This study was conducted by Dr. Nagel from Auburn University in the United States.
Reference:
Nagel et al.
Salmonella and Campylobacter reduction and quality characteristics of poultry carcasses treated with various antimicrobials in a post-chill immersion tank
International Journal of Food Microbiology 165 (2013) 281–286
Dr. Nagel’s research involved treating 160 broilers with different disinfectants during the chilling process. The disinfectants tested were chlorine (40 ppm), peracetic acid (400 ppm and 1000 ppm), and lysozyme (1000 ppm and 5000 ppm). The aim was to determine the effectiveness of these treatments in reducing Salmonella and Campylobacter. Chlorine at 40 ppm was used as a control because it is widely employed in the broiler industry. Lysozyme was included due to its prior use in poultry chilling and its reputation as a harmless, egg-derived antimicrobial agent.
Results: Reduction of Salmonella and Campylobacter
The study found that peracetic acid, at both 400 ppm and 1000 ppm, was the most effective disinfectant in reducing Salmonella and Campylobacter levels.
In the United States, chlorine and organic acids have traditionally been the primary disinfectants used during the chilling process. However, the effectiveness of chlorine significantly diminishes at higher pH levels.
For those interested in the basics of chlorine disinfection and its relationship with pH, please refer to this article: "Food Factories - Sodium Hypochlorite".
Organic acids are effective at preventing pathogens from adhering to the meat surface. However, once pathogens are attached, their effectiveness decreases. Furthermore, the use of organic acids may negatively affect the sensory qualities of the chicken, a concern that has been widely reported.
As a result, there is a growing need to combine other disinfection mechanisms with organic acids, preferably without resorting to high concentrations. Peracetic acid represents a prime example of this alternative approach.
How Peracetic Acid Works
Peracetic acid combines the antimicrobial properties of organic acids with the oxidative effects of hydrogen peroxide. Upon decomposition, it breaks down into acetic acid and hydrogen peroxide, both of which contribute to its bactericidal action.
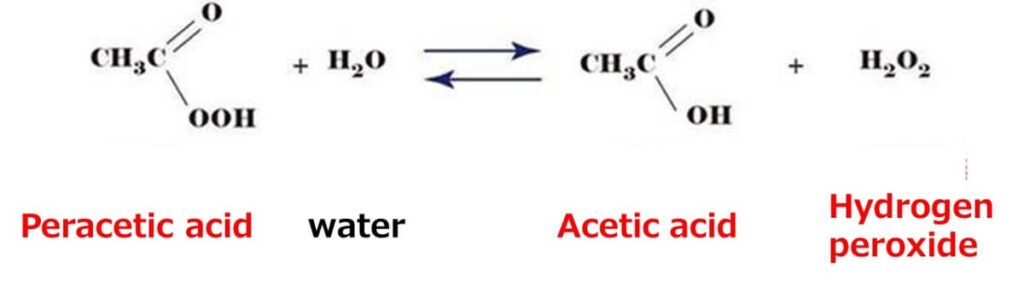
While the detailed mechanisms of its antimicrobial action are not yet fully understood, peracetic acid broadly acts through a combination of acidic stress and oxidative damage from reactive oxygen species.
My Take on This Study

What struck me most in this study is the clear demonstration that peracetic acid outperforms both chlorine and lysozyme in reducing Salmonella and Campylobacter during the broiler chilling process. From my perspective, the key takeaway is that peracetic acid, even at relatively moderate concentrations (400–1000 ppm), achieves superior microbial control without relying on the drawbacks of chlorine, such as pH sensitivity or sensory degradation from organic acids.
For food safety professionals, this has practical implications: it supports the use of peracetic acid as a viable and potentially superior alternative to traditional disinfectants in poultry processing. Notably, peracetic acid is already approved in the United States for use in broiler chilling tanks up to 2000 ppm and was approved as a food additive in Japan in 2016.
This aligns with what I have seen in food industry settings—effective antimicrobial agents must strike a balance between efficacy, safety, and impact on product quality. Peracetic acid appears to meet all three, and this study offers strong evidence to support its adoption in HACCP-based disinfection protocols.
For those interested in the basics of peracetic acid, please refer to :
The Role of Peroxyacetic Acid Formulations in Food Factory Disinfection