Discover how peroxyacetic acid formulations stand out as robust disinfectants in food factories, especially for their resistance to organic matter. This article delves into their unique properties, production process, and approved applications in food safety.
Peroxyacetic Acid's Resistance to Organic Matter
Peroxyacetic acid’s most remarkable feature is its ability to retain disinfecting power even in environments rich with organic matter.

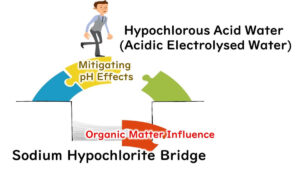
In the case of hypochlorous acid, organic materials on surfaces often consume active oxygen, reducing its effectiveness against microbes. This drawback makes thorough cleaning a prerequisite before disinfection when using hypochlorous acid.
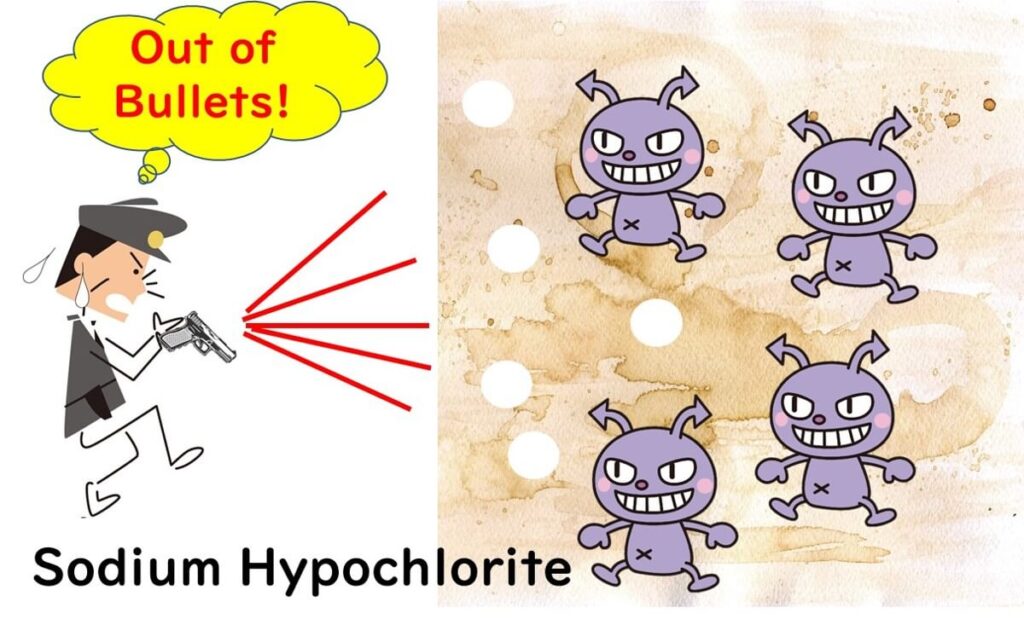
On the other hand, peroxyacetic acid is far less affected by organic substances, allowing it to maintain its microbe-killing efficiency even without perfect pre-cleaning.

How Peroxyacetic Acid is Made
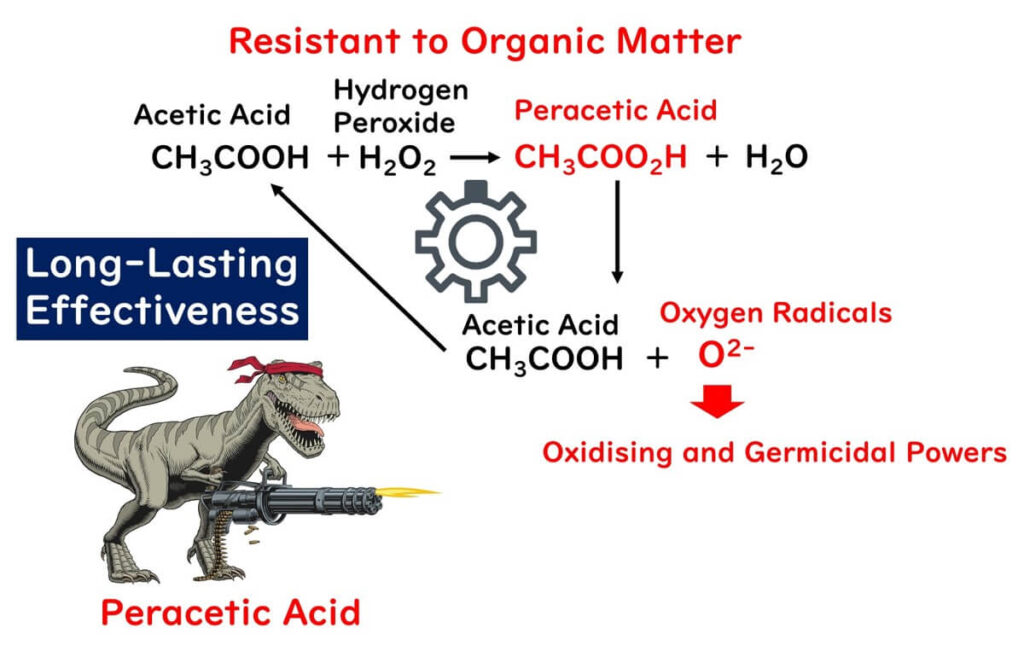
The resilience of peroxyacetic acid to organic matter lies in its unique production process. It is synthesized by adding hydrogen peroxide to acetic acid, resulting in the formation of peroxyacetic acid. Once formed, peroxyacetic acid dissociates into acetic acid and oxygen radicals, which are responsible for its powerful oxidizing effect.
Interestingly, the acetic acid produced in this reaction can revert to the initial acetic acid used for synthesizing peroxyacetic acid, creating a self-sustaining cycle. This cycle ensures that the disinfecting power of peroxyacetic acid remains consistent, even in the presence of substantial organic material.
Standards for Peroxyacetic Acid Formulations
Peroxyacetic acid is widely recognized as a disinfectant across the globe, though its applications are subject to varying regulations. In Japan, it is commonly utilized for cleaning equipment and production lines in food factories. Since 2016, its use has been extended to surface disinfection for meat and vegetables, emphasizing its versatility and regulatory acceptance.