Dive into the intriguing world of sodium hypochlorite, a cornerstone of disinfection in food factories. This comprehensive guide illuminates the science behind its germ-fighting prowess and navigates through its strengths and weaknesses in a battle against contaminants. From its chemical makeup to the crucial role pH levels and organic matter play, uncover the nuanced dynamics that make sodium hypochlorite a hero in cleanliness and safety in our food production lines. Join us as we decode the secrets of this ubiquitous disinfectant, ensuring you're well-versed in the essentials of food microbiology and the safeguarding measures that keep our meals safe.
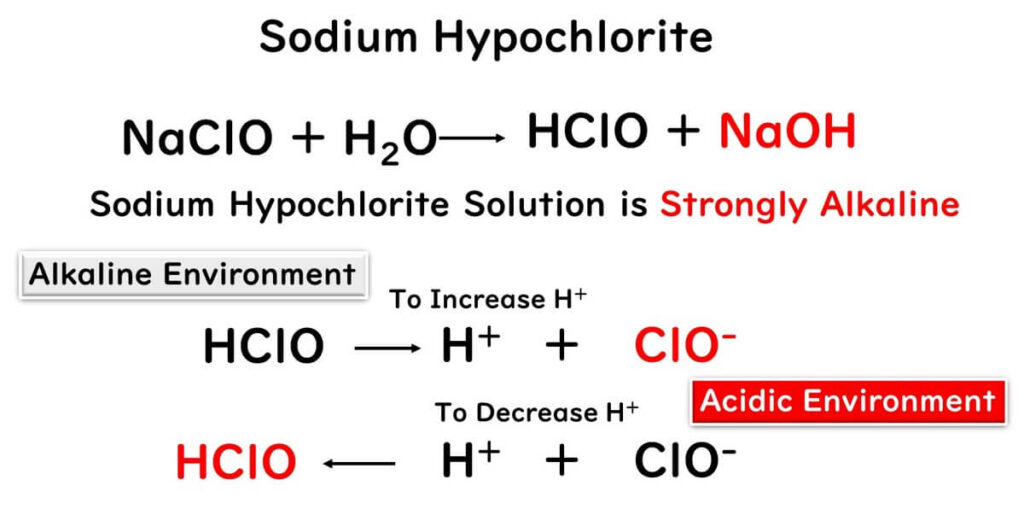
Sodium Hypochlorite Solution is Weakly Alkaline
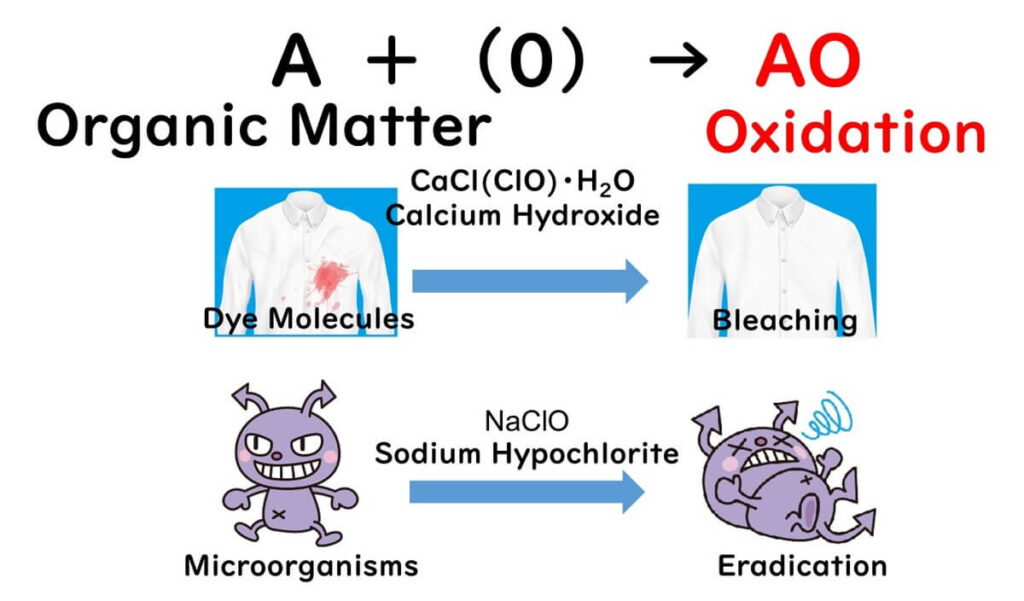
Let's dive into the world of chlorine-based disinfectants, the unsung heroes in the battleground against germs, particularly focusing on sodium hypochlorite. The secret weapon of chlorine disinfection lies in its power to oxidize organic matter. It's akin to the bleach used for your clothes, which employs calcium hydroxide, sharing the same principle of attacking organic materials with active oxygen.
In the realm of food factories, sodium hypochlorite takes the crown as the go-to chlorine-based disinfectant. This chemical, a blend of HCLO and sodium hydroxide, swings towards the alkaline end of the pH scale. However, an alkaline environment doesn't quite roll out the red carpet for its oxidizing power. Why? In such conditions, HCLO transforms mostly into CLO-, diluting its oxidizing strength, which directly correlates with the concentration of HCLO. The peak of HCLO's prowess is witnessed at a pH range of 5 to 6. Even diluting sodium hypochlorite to its limits won't nudge the pH down to this ideal window.
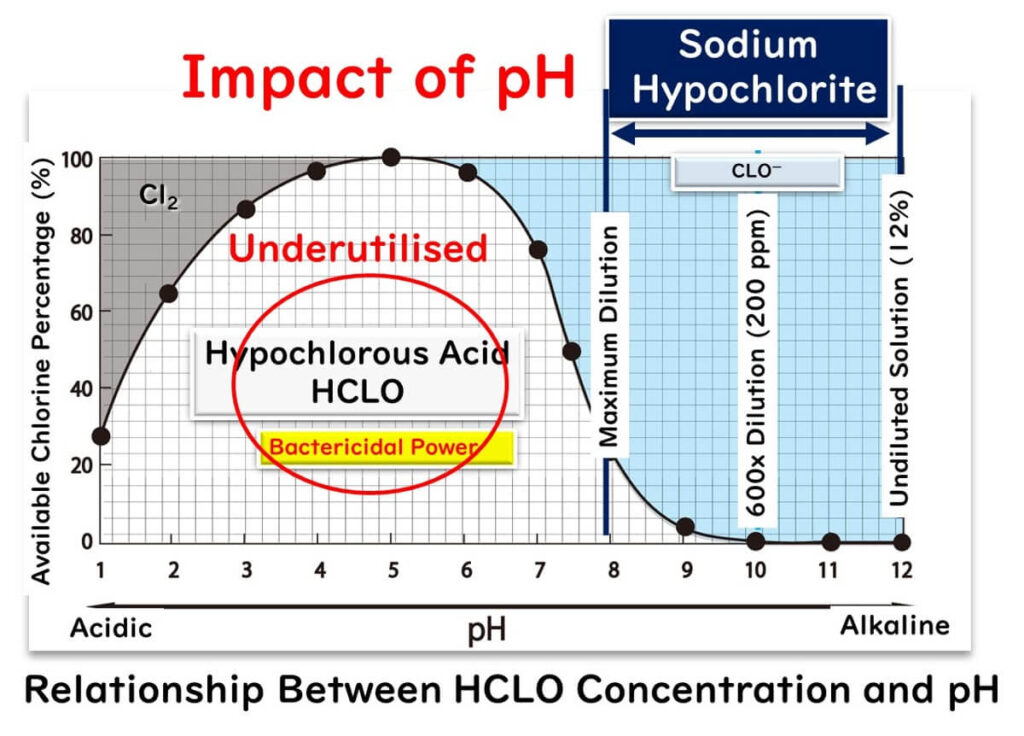
Why Does Disinfection Power Dip Outside of Acidic Conditions?
Under acidic conditions, HCLO flourishes, bringing its potent disinfection power to the forefront. On the flip side, CLO-’s disinfection game is weak, sitting at roughly 1/80th the strength of HCLO. So, a mere aqueous solution of sodium hypochlorite isn't cutting the mustard in terms of oxidizing power. Why is that, you ask?
Peeling back the layers, we need to understand the basic nature of microbial cell membranes. The hydrophobic lipid bilayer, forming the skeleton of these membranes, lets hydrophobic compounds slip through easily. However, charged ions like hydrogen ions get the cold shoulder, unable to cross the lipid double whammy.
The Lowdown on What Can and Can't Cross the Cell Membrane
Understanding which substances breeze through the hydrophobic lipid bilayer and which get the boot is crucial. This knowledge isn’t just pivotal for grasping how sodium hypochlorite works its magic but is also a cornerstone concept in the field of food microbiology, especially when discussing microbial disinfection and growth inhibition.
- Hydrophobic substances can traverse the lipid bilayer. This includes meds or naturally occurring gases like carbon dioxide and oxygen.
- Polar molecules generally get a thumbs down from the hydrophobic (lipid bilayer) doorman. However, if these polar molecules are small enough, they might sneak in. Water molecules, for example.
- Larger polar molecules are shown the exit, unable to pass through.
- Compounds, regardless of being positively or negatively charged, are barred entry, irrespective of size. This includes small ions like hydrogen ions—a critical point to understand in various aspects of food microbiology. Naturally, large charged substances like amino acids also can't make the cut.
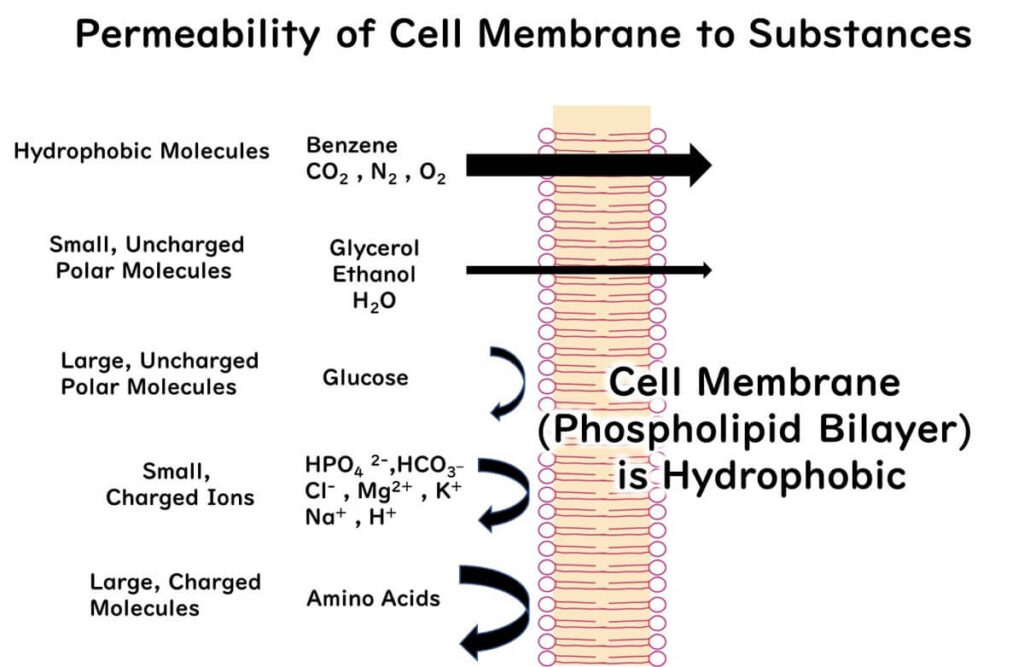
Why Sodium Hypochlorite Can Slip Through the Cell Membrane
Swinging back to the disinfecting power of sodium hypochlorite, HCLO, though a polar molecule, is small enough to just about slip through the cell membrane. In contrast, CLO-, being charged, is stopped in its tracks. This difference in membrane permeability is where the disinfecting power varies significantly. Essentially, HCLO can waltz into the cell and oxidize the internal substances, while CLO- gets left outside, limiting its disinfecting potential.
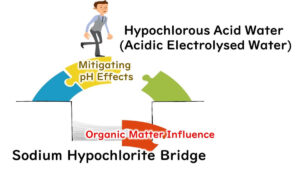
Adding an acid like hydrochloric acid to lower the pH could theoretically boost sodium hypochlorite’s disinfecting power. However, get the mix wrong, and you're in a world of danger, risking the release of toxic chlorine gas—a definite no-go in practice.

Vulnerable to Organic Matter
Another point to ponder is that chlorine-based disinfectants, being oxidizers, don't exclusively target microbes.

If organic matter is in the vicinity, it gets oxidized too. Without thorough cleaning to remove dirt and grime, the disinfecting power of sodium hypochlorite could be gobbled up by organic matter.
This is particularly crucial when washing vegetables, which are essentially lumps of organic matter, meaning most of the oxidizing power may end up targeting the veggies themselves. However, in a squeaky clean factory setting, sodium hypochlorite's oxidizing power can focus on microbes, maximizing its disinfecting potential.
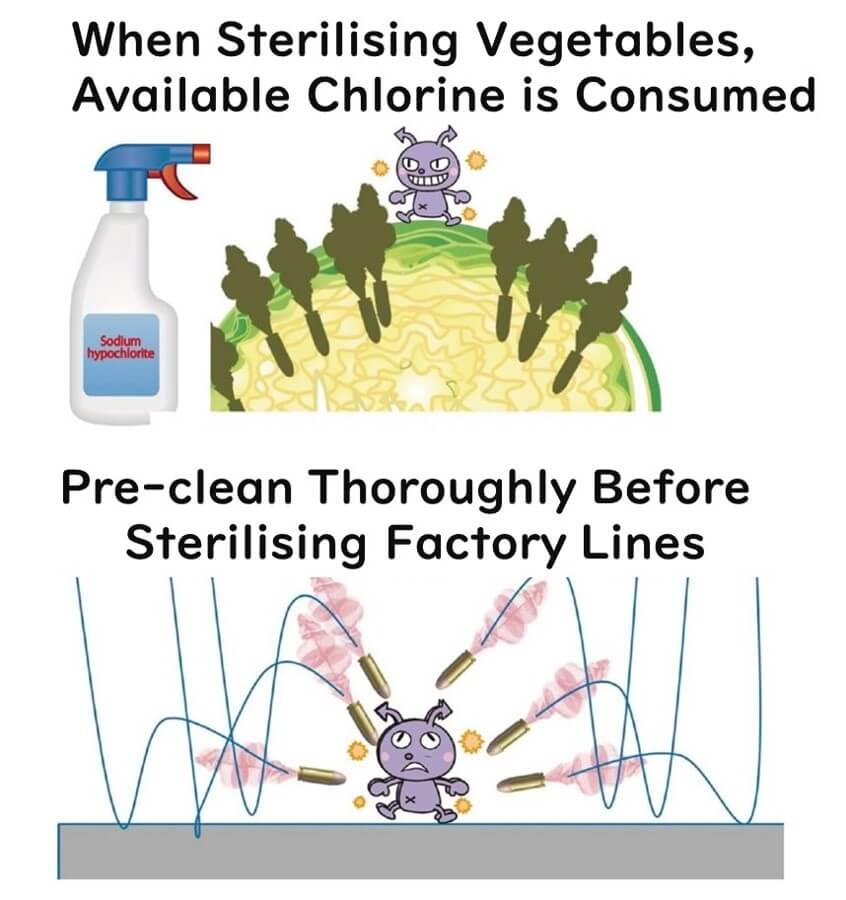
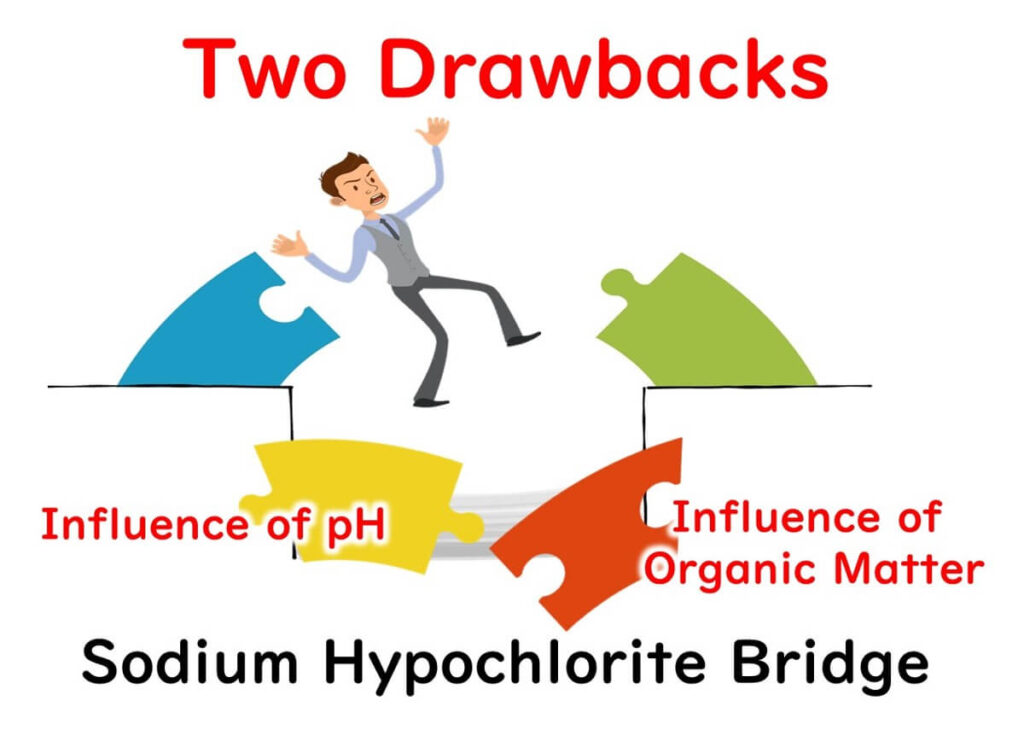
Two Achilles' Heels of Sodium Hypochlorite
Despite its widespread use in food factories worldwide for its simplicity and cost-effectiveness, sodium hypochlorite is not without its flaws. Its two main drawbacks are 1) its susceptibility to pH changes and 2) its vulnerability to organic matter. The next section will outline disinfectants that overcome these shortcomings of sodium hypochlorite.