This article summarizes the basics of ozone gas and ozone water. Ozone, a potent oxidizing agent, boasts remarkable sterilizing power. Additionally, it naturally decomposes into non-toxic oxygen, leaving no residues when used as a food sterilizer. Ozone can be utilized in two forms: as ozone gas or ozone water. Using ozone gas requires high concentrations, but it comes with several challenges, such as toxicity due to gas diffusion and the corrosive effect it has on a wide range of equipment, limiting its current applications in industry. On the other hand, ozone water, even in minimal concentrations (1-5 ppm), exhibits antimicrobial activity. The use of ozone water is not only promising for the food industry but also has broad potential applications in domestic and medical sectors.
Ozone Gas
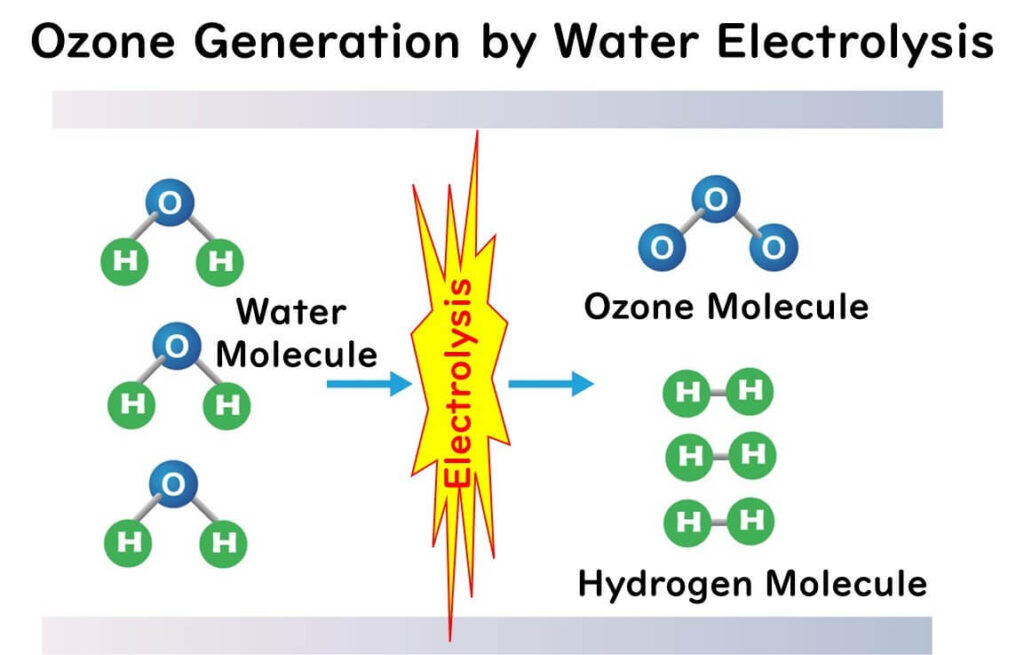
Ozone, denoted chemically as "O3," is a molecule consisting of three oxygen atoms. The term "ozone" originated around 1840 when Christian Friedrich Schönbein noted the distinctive smell of the gas produced by electrolysis of water and named it from the Greek word "ozein" (to smell). Ozone has a molecular weight of 48 and is 1.7 times denser than air.
Ozone's strong oxidizing properties endow it with broad antimicrobial capabilities against bacteria, bacterial spores, fungi, and viruses. As such, ozone has been a long-standing choice for water treatment.
zone Gas Generation in the Atmosphere
There exists a protective layer known as the ozone layer approximately 25 km above Earth's surface, safeguarding life from the harmful ultraviolet radiation emitted by the sun. This ozone layer did not exist 3.3 billion years ago when life first emerged on Earth. About 1 billion years after the advent of life, photosynthesizing cyanobacteria emerged, leading to the formation of the ozone layer. The creation of this layer enabled life to shield itself from the intense ultraviolet rays of the sun, eventually facilitating the migration of life onto land.

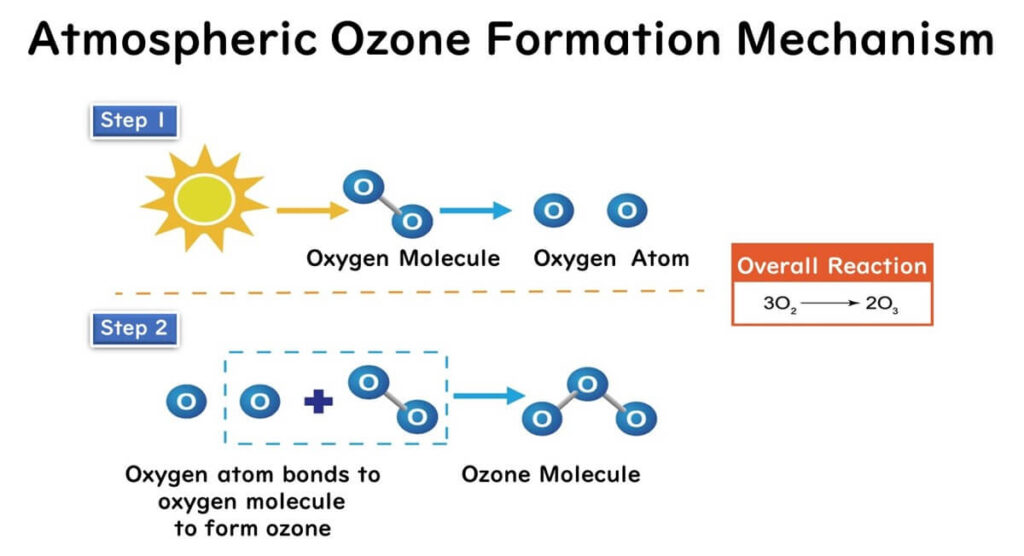
The mechanism of ozone formation in the atmosphere due to sunlight is as follows:
- Oxygen molecules in the atmosphere are split into oxygen atoms when struck by the sun's ultraviolet rays.
- These oxygen atoms then combine with other oxygen molecules to form ozone molecules, composed of three oxygen atoms.
Artificial Generation Methods
When synthesizing ozone artificially, the process involves splitting oxygen molecules into two oxygen atoms, which then react with another oxygen molecule, resulting in the reaction O + O2 → O3.
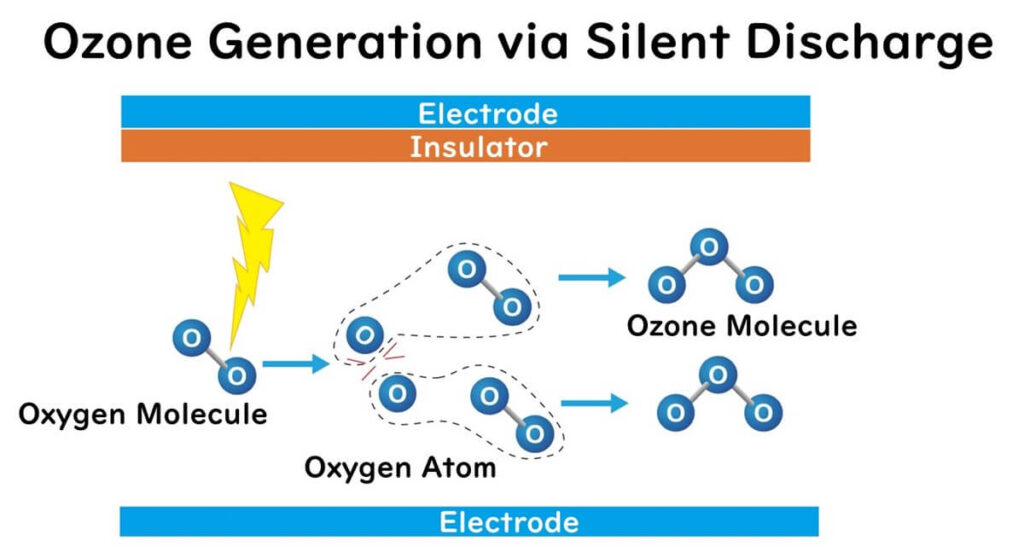
Silent Discharge
This method is a prevalent technique for generating large amounts of ozone gas. One or both electrodes are covered with an insulator, and an alternating current voltage is applied, causing a discharge. It is termed "silent" because the electrodes are insulated, preventing the flow of charge to the electrodes and thus avoiding large currents. Consequently, unlike corona discharge, no sound is produced during discharge.
When air or oxygen is passed through during silent discharge, ozone is generated. High-energy discharges break down oxygen molecules into atomic oxygen radicals, which spontaneously combine with oxygen molecules to form ozone molecules.
Ultraviolet Irradiation Method
This method involves irradiating gases containing oxygen (typically air) with ultraviolet light to generate ozone. As mentioned earlier, this artificially replicates the natural process that forms the ozone layer in the stratosphere.
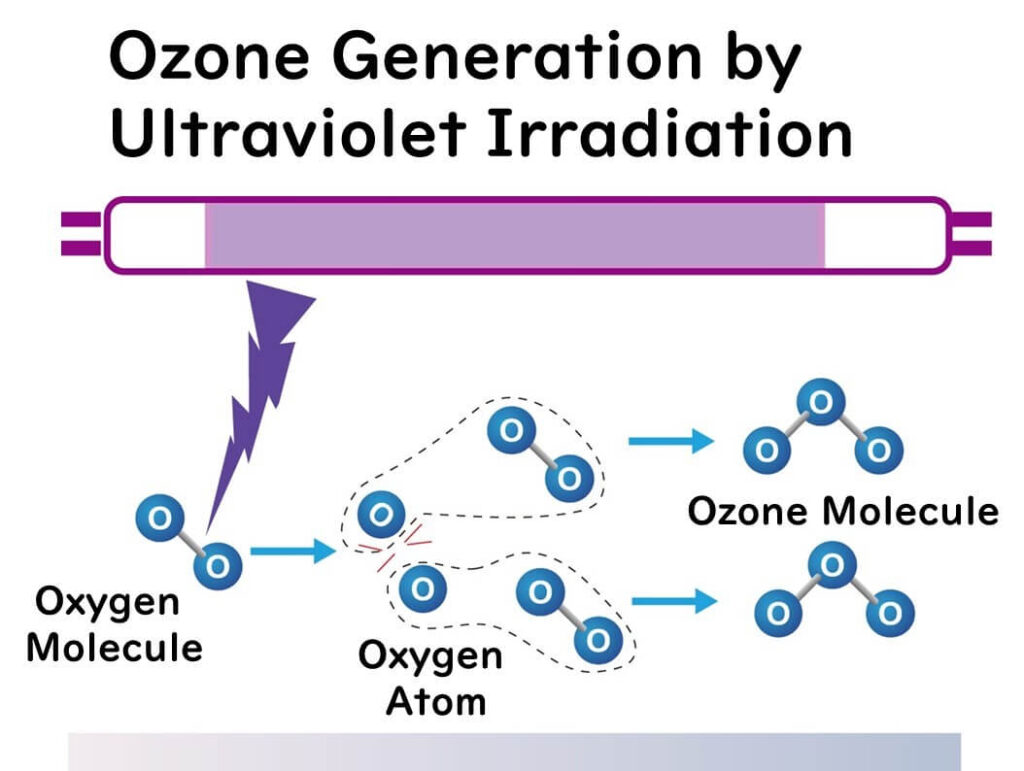
When ultraviolet light is shone on oxygen molecules, they dissociate into oxygen atoms, which then combine with oxygen molecules to produce ozone. However, ozone molecules are also easily decomposed by ultraviolet light. Therefore, while the ultraviolet irradiation method cannot generate large amounts of ozone, it is beneficial for its simplicity, making it suitable for air sterilization, deodorization, and small-scale water sterilization.
Electrolysis Method
This method generates ozone by electrolyzing solutions of sulfuric acid or hydrochloric acid. It requires large-scale equipment but can produce ozone concentrations of 15-20w%, making it suitable for generating high-concentration ozone water. However, compared to the silent discharge method, the ozone generation efficiency of the electrolysis method is lower, making it less suitable for large-scale applications.
Sterilization Mechanism
When considering the sterilization mechanism of ozone gas, it is useful to understand the mechanism of chlorine sterilization. Both ozone and chlorine sterilization share a common mechanism, which fundamentally involves the oxidizing action of reactive oxygen species.
For an accessible explanation of chlorine sterilization, please refer to the following article:
Food Factories - Sodium Hypochlorite
Ozone molecules (O3) have weaker atomic bonds compared to oxygen molecules (O2), making them unstable and prone to decomposition. This characteristic allows ozone to easily split into oxygen (O2) and atomic oxygen (O). The atomic oxygen produced by the decomposition of ozone possesses very strong oxidizing power. This reactive oxygen species can oxidize organic matter and sterilize microbes.
The primary action mechanism of ozone, particularly reactive oxygen species, is lipid peroxidation, which damages membrane phospholipids at the cellular level. Additionally, ozone oxidizes amino acids, irreversibly altering the structure and function of proteins.
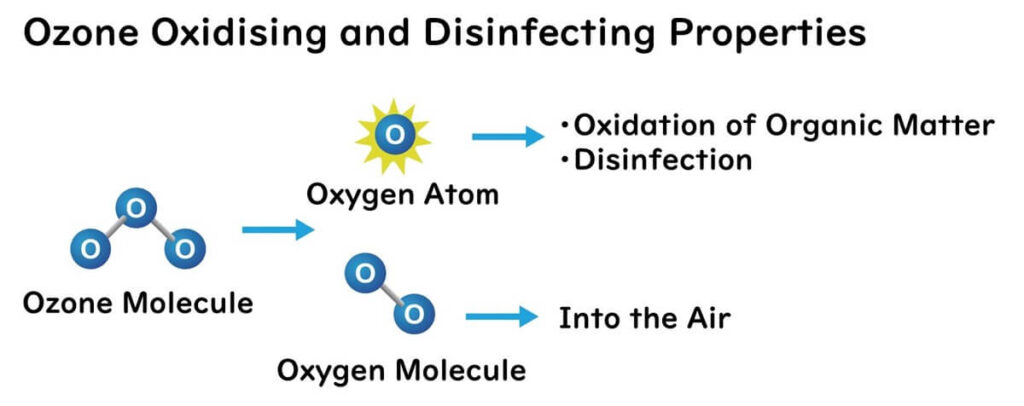
Moreover, ozone molecules, like oxygen, have hydrophobic properties. Therefore, some ozone gas can pass through the phospholipid bilayer of bacterial cell membranes. As a result, ozone gas is believed to be able to oxidize proteins and DNA within cells, causing damage to microbial cells.
For an easy-to-understand explanation of substances that can easily pass through microbial phospholipid bilayers, please see the following article:
The Lowdown on What Can and Can't Cross the Cell Membrane
Practical Applications
Most research focuses on the sterilizing effects of ozone water, while studies on the use of gaseous ozone are more limited.
As a Sterilizing Agent for Fruits
Reports indicate that applying 50,000 ppm of ozone to strawberries and blueberries inoculated with Salmonella and E. coli O157:H7 can reduce the number of these pathogens by 3 log cfu (as per literature). Additionally, treating cantaloupes with 10,000 ppm of ozone gas for 30 minutes controlled E. coli O157:H7 without adversely affecting the fruit's quality (Selma et al., 2007).

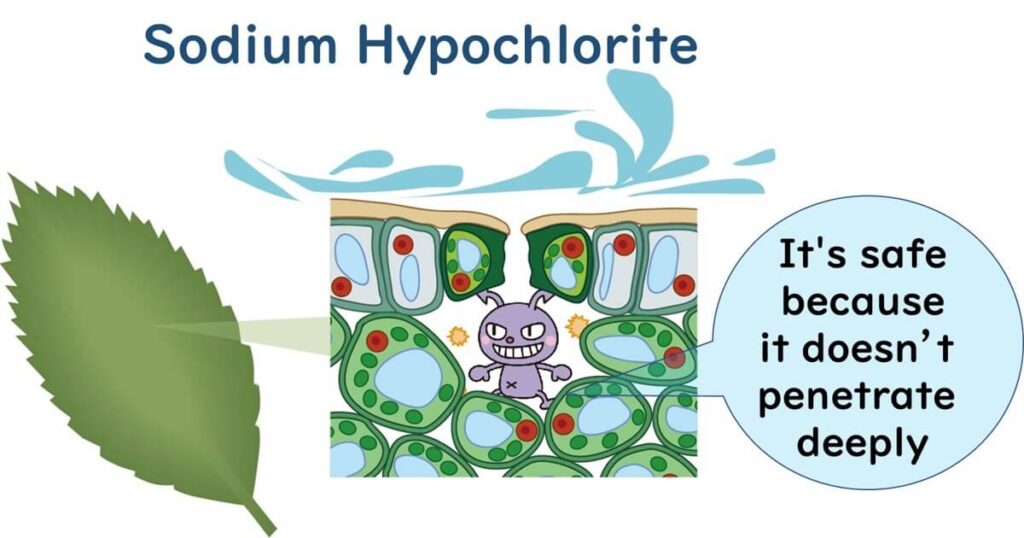
As a Sterilizing Agent Penetrating Vegetables
Typically, chlorine in the form of sodium hypochlorite or calcium hypochlorite is used to wash fresh produce like vegetables. However, pathogens such as E. coli O157:H7 and Salmonella are known to be absorbed into leafy vegetables. Eliminating pathogens that have penetrated the interiors of plants with sodium hypochlorite is challenging. Reports have noted the incorporation of such enteric pathogens in edible plants including tomatoes, radish sprouts, and lettuce.
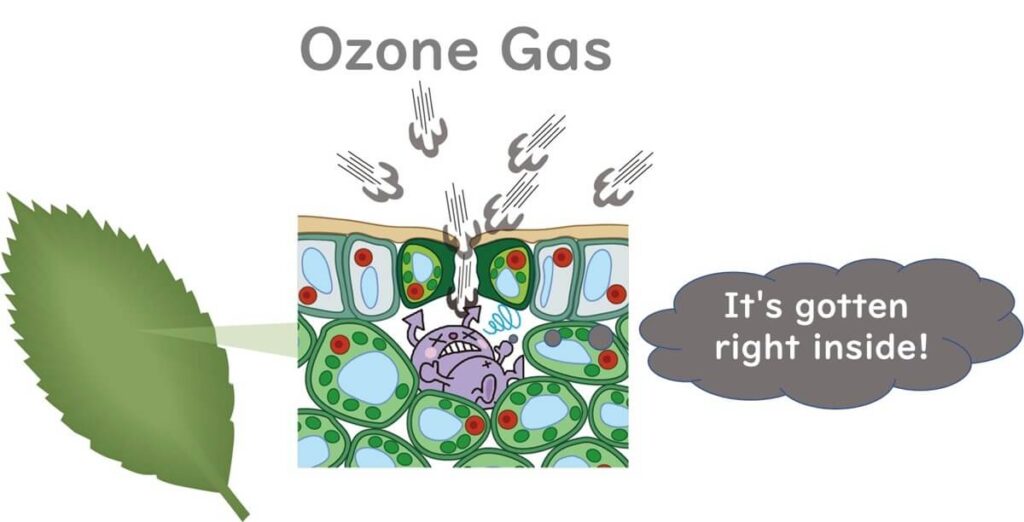
Attempts are also being made to use ozone gas to remove pathogens that have infiltrated the interiors of leafy vegetables. For instance, an experiment applied ozone gas to baby spinach contaminated with E. coli O157:H7(Vurma et al., 2009). Initially, the spinach was vacuum cooled, and then treated with 5-10 ppm ozone gas, significantly facilitating the diffusion of the gaseous sterilant into the leafy greens. This study showed a reduction of 1.8 log CFU/g of E. coli O157:H7.
As an Air Decontaminant in Factory Environments
Ozone gas is considered a method for sterilizing aerosols floating in the air environment of factories. The advantage of ozone is that, being a gas, it can easily sterilize areas like ceilings and walls where liquid sterilants cannot reach. Currently, the use of ozone gas for such purposes is practiced in some pharmaceutical and clinical sectors.
Although it's challenging to say that ozone gas is widely used for such purposes in food processing environments at present, it is beginning to be utilized in some areas.
For example, in Italy's natural cheese industry, air contamination by mold in manufacturing plants significantly damages cheese products. The Italian Ministry of Health (2010) has issued a statement supporting the use of gaseous ozone for disinfecting cheese aging and storage facilities.
For effective implementation of ozone gas in food processing plants, ensuring a closed environment is crucial. Measures such as administering ozone at night or on weekends to prevent workers from being exposed to large amounts of ozone are necessary. In the United States, the Occupational Safety and Health Administration (OSHA) recommends that ozone exposure not exceed 0.1 ppm (corresponding to 0.2 mg/m³ in air) under normal working conditions for 8 hours a day or 40 hours a week to prevent adverse effects.
Drawbacks of Ozone Gas
While ozone gas is a powerful sterilizing agent, it also has several drawbacks:
- Irritation and Toxicity: Ozone gas can irritate the eyes and throat and is toxic to the respiratory system. Exposure to concentrations over 4 ppm for prolonged periods can be lethal to humans. Ozone should never be used in rooms or areas where inhalation is possible, and its use as a disinfectant in environments where human activities are conducted is severely limited.
- Containment Challenges: It is often difficult to contain the gas in the target environment, which can limit its practical applications.

- Corrosive Nature: Due to its broad diffusion and strong oxidizing power, ozone gas can cause extensive corrosion to metal surfaces, such as those found in fans and condenser coils.
- Instability and Rapid Decomposition: Ozone is unstable and decomposes quickly, which means it must be generated on-site as needed—a drawback it shares with ozone water.
- Reduced Efficacy in the Presence of Organic Matter: Like other oxidants, the effectiveness of ozone decreases in the presence of organic materials, which can absorb and neutralize the ozone before it can act on intended targets.
Ozone Water
Ozone water is created by dissolving ozone gas into water. The dissolved ozone reacts with OH- in the water to produce highly oxidizing agents such as OH (hydroxyl radicals) and O2- (superoxide ions). Notably, the hydroxyl radical has a higher oxidation potential than ozone itself. Hence, ozone water possesses a robust oxidizing capability. Using ozone in water allows for disinfecting effects at lower concentrations than when used as a gas, typically ranging from 0.1ppm to 50ppm.
Compared to ozone gas, ozone water finds wide applications including in the food industry. It's utilized in various settings like wastewater treatment facilities, dairy and swine effluent, cooling towers, hospital water systems and devices, aquariums and aquaculture, water theme parks, as well as public and domestic spas.
Production Methods
There are generally two main methods for generating ozone water.
Gas Dissolution Method
As mentioned, this method involves dissolving purified ozone gas, created via silent discharge, into tap water using specific equipment. This setup tends to be large and complex.
This method is primarily used in large facilities such as water purification plants.
Electric Field Method
This method involves electrolyzing water with electrodes to directly generate ozone water. It is often employed in small household appliances as an ozone water generator. Recently, devices that electrolyze tap water directly without the need for pure water are also available on the market.
This cheeky journey into the world of ozone water shows how something as airy as ozone can be pinned down in water to clean more than just your typical fish tank! A bit of a scientific marvel, wouldn't you say?
Advantages of Ozone Water
- Easy Home Production: With a small device, anyone can produce ozone water from tap water at home, eliminating the need for transportation or stockpiling.
- Zero Waste in Production: Unlike ethanol or soap, which generate substantial waste from distribution and packaging, ozone gas production results in no waste.
- Lower Effective Antimicrobial Concentration: The effective antimicrobial concentration of ozone is significantly lower than that of sodium hypochlorite.
- Safe Decomposition: Ozone does not produce toxic byproducts in typical reactions and eventually decomposes back into oxygen, eliminating health risks.
Disadvantages of Ozone Water
- Instability in Water: Ozone is highly unstable in water, decomposing into oxygen within a short time (more than half is inactive after 20 minutes). In polluted water containing dissolved organics, the half-life of ozone activity can be less than a minute.
- Difficulty Maintaining Effective Concentrations: Unlike chlorine or chlorine dioxide, it is challenging to generate ozone remotely and inject it into a centralized water supply system to maintain effective microbial disinfection concentrations. However, this drawback is mitigated by the ease of producing ozone water once the equipment is purchased.
- Corrosiveness: Similar to other oxidizers like sodium hypochlorite, ozone has the potential to corrode metals.
Cheerio! While ozone water sounds like a superhero in a bottle—swift, clean, and disappearing without a trace—it's not without its kryptonite, especially when it comes to stability and metal friendships. But, as with all good tales, its strengths often shine through the clouds!
Examples of Applications
Ozone water is widely used in various settings such as wastewater treatment facilities, livestock operations, agricultural sites, industrial facilities, and for pool disinfection. This article highlights particularly relevant research on the disinfection of alfalfa vegetables from Salmonella and enterohemorrhagic Escherichia coli.
Disinfection of Alfalfa Seeds and Sprouts
In the United States, between 2010 and 2017, there were a total of 15 multistate outbreaks related to the consumption of sprouts. Out of these outbreaks, 12 were caused by sprouts contaminated with Salmonella Enterica, and three were due to contamination with enterohemorrhagic E. coli.
The study introduced below inoculated alfalfa seeds with a cocktail of three strains of Salmonella (serotypes Typhimurium, Agona, Saintpaul) and three strains of enterohemorrhagic E. coli (serotypes O104:H4, O157:H7, O121:H19) at a concentration of 7.0 log CFU/ml. The inoculated seeds and the sprouts grown from them were then immersed in 1 liter of ozone water containing 5 mg/L (5 ppm) at 5°C for durations of 0 (before immersion), 10, 15, or 20 minutes.
Salmonella
- The average logarithmic reductions of Salmonella obtained from the seeds after 10, 15, and 20 minutes of ozone water treatment were 1.6, 1.7, and 2.1, respectively.
- For the sprouts, the reductions were 0.7, 1.1, and 3.6, respectively.
Enterohemorrhagic E. coli
- For the seeds, the average logarithmic reductions after 10, 15, and 20 minutes of ozone water treatment were 1.5, 1.6, and 2.1, respectively.
- The study confirmed that low concentration (5 mg/L) ozone treatment can significantly reduce Salmonella and enterohemorrhagic E. coli. This method could be a promising intervention to decrease Salmonella and STEC from alfalfa seeds and sprouts.
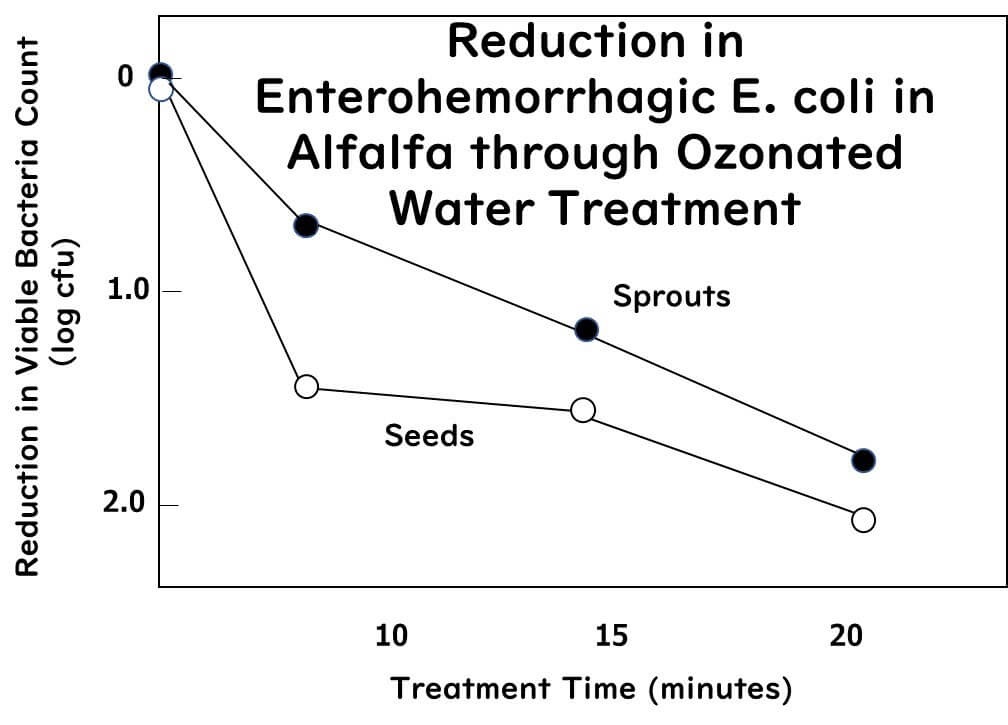
The diagram above is drawn from some of the data from the literature presented here.
Reduction of Salmonella and Shiga toxin-producing Escherichia coli on alfalfa seeds and sprouts using an ozone generating system
International Journal of Food Microbiology, 289, 16 , 57-63 (2019)
Synergistic Effects of Ozone Water and Acidic Electrolyzed Water
Since neither ozone water nor electrolyzed water alone can completely remove all pathogens from the surface of alfalfa seeds and sprouts (Mohammad et al., 2019; Liu and Yu , 2016), this research explored the use of a combined treatment of ozone water and acidic (pH 3.0) electrolyzed water.
Alfalfa seeds inoculated with a cocktail of three Salmonella strains and three enterohemorrhagic E. coli strains were sequentially treated with ozone water and acidic electrolyzed water. The treatment involved immersing the samples in ozone water (5 mg/L ozone) for 15 or 20 minutes, followed by a 15-minute immersion in 1 liter of acidic electrolyzed water. The results were as follows:
- The combined treatment (ozone + electrolyzed water) showed higher reduction effects than either treatment alone.
- The combined treatment achieved reductions of 3.6 and 2.9 log CFU/g for Salmonella and enterohemorrhagic E. coli in the seeds, respectively, and 3.1 and 3.0 log CFU/g in the sprouts.
- The combined ozone and acidic electrolyzed water treatment had no adverse effects on the appearance quality of the sprouts.
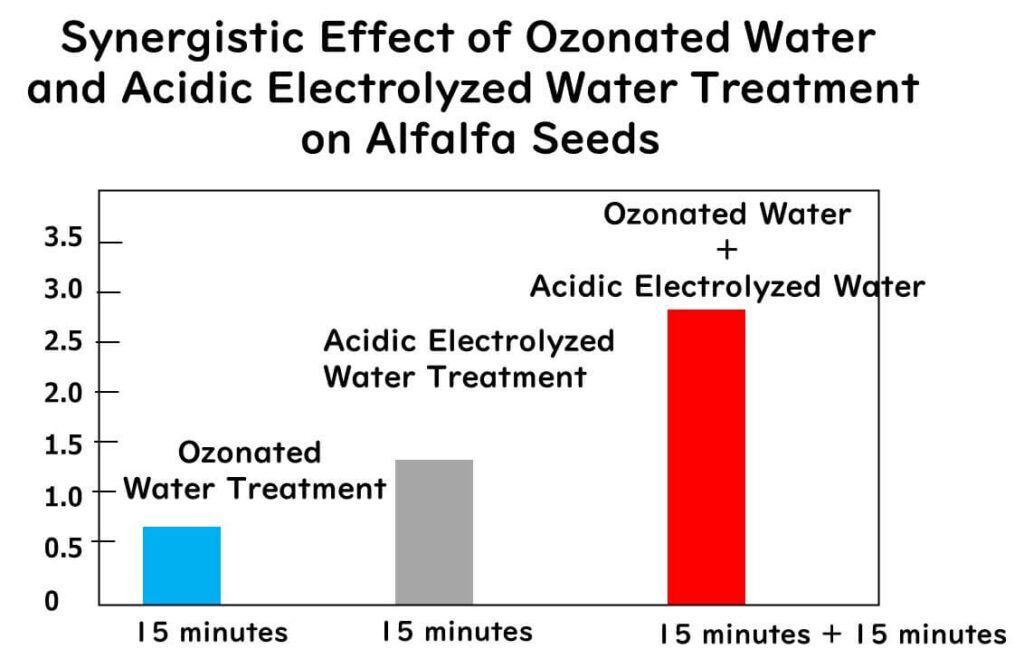
The diagram above is drawn from some of the data from the literature presented here.
The combination of ozone and acidic electrolyzed water is effective for inactivating Salmonella and enterohemorrhagic E. coli on alfalfa seeds and sprouts without compromising the quality of the sprouts.
Inactivation of Salmonella and Shiga toxin-producing Escherichia coli (STEC) from the surface of alfalfa seeds and sprouts by combined antimicrobial treatments using ozone and electrolyzed water
Food Research International 136, 109488(2020)
Other Applications
The use of ozone water is also increasing in dental clinics for sterilizing oral care products and instruments. Additionally, a growing number of household devices are being sold.
Recently, researchers in a Norwegian hospital have recommended ozone water for washing nurses' hands, noting that it has the same sanitizing effect as alcohol-based sanitizers but causes less skin irritation. More details on this study are compiled in a separate article.