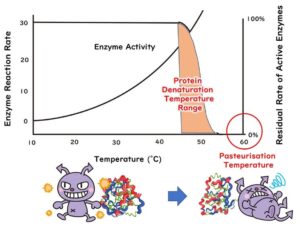
Ensuring the safety of food through heat sterilisation is crucial. This article explores the fundamentals of two common methods: moist heat sterilisation and dry heat sterilisation. We discuss their differing efficiencies against microbes, focusing on how environmental moisture influences sterilisation effectiveness and microbial survival. Additionally, we examine heat-resistant spore-forming microbes and the scientific reasons behind their resistance to standard sterilisation techniques, providing insights from microbiological principles to enhance the understanding of food sterilisation processes.
Why Is There Such a Difference in Sterilisation Efficiency Between Moist and Dry Heat?
When considering heat sterilisation of microbes, the relationship between sterilisation efficiency and moisture content is a fundamental principle. In basic microbiology experiments, we often encounter two key methods: autoclaving (moist heat sterilisation) and dry heat sterilisation.
Autoclaves utilise steam under pressure to sterilise items such as microbial culture media, which contain moisture. This process typically operates at 120°C for about 20 minutes. In contrast, dry heat sterilisation is commonly used for sterilising glassware or metal equipment. This method requires higher temperatures, around 160°C, and significantly longer durations, typically around an hour.
Despite both being sterilisation methods, the longer time and higher temperatures needed for dry heat sterilisation highlight the critical role of moisture in enhancing sterilisation efficiency. This distinction underscores the importance of selecting the appropriate method based on the items being sterilised and their resistance to heat.

Why Does Dry Heat Sterilisation Require Higher Temperatures and Longer Times Than Moist Heat Sterilisation?
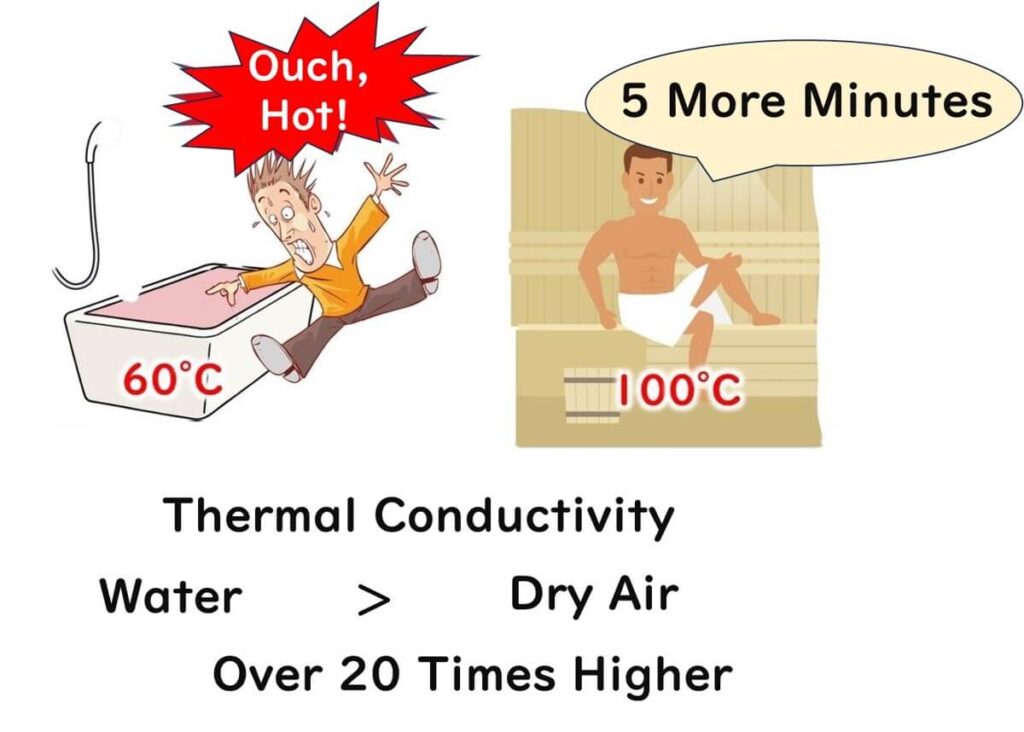
Why does dry heat sterilisation demand significantly higher temperatures and longer times compared to moist heat sterilisation? Let’s explore this concept through a relatable example.
Imagine dipping your finger into a bath of hot water at 60°C. The heat would feel intense, as 60°C is sufficient to denature proteins. Now, consider sitting in a sauna with an air temperature of 100°C. Surprisingly, you wouldn’t feel as much heat despite the higher temperature. Why? The difference lies in the thermal conductivity of water and air.
Water has a thermal conductivity of 0.582, while air's is only 0.0241, making water 25 times more efficient at transferring heat. This is why hot water quickly heats your finger, whereas air in a sauna transfers heat more slowly. For microbes, a moisture-rich environment during moist heat sterilisation functions like a hot water bath: heat penetrates efficiently, denaturing proteins and killing the microbes.
In contrast, dry heat sterilisation resembles the sauna environment. Even at 160°C, the dry air transfers heat more slowly, allowing microbes to withstand the high temperature longer. This explains why moisture content plays a critical role in the efficiency of sterilisation methods.
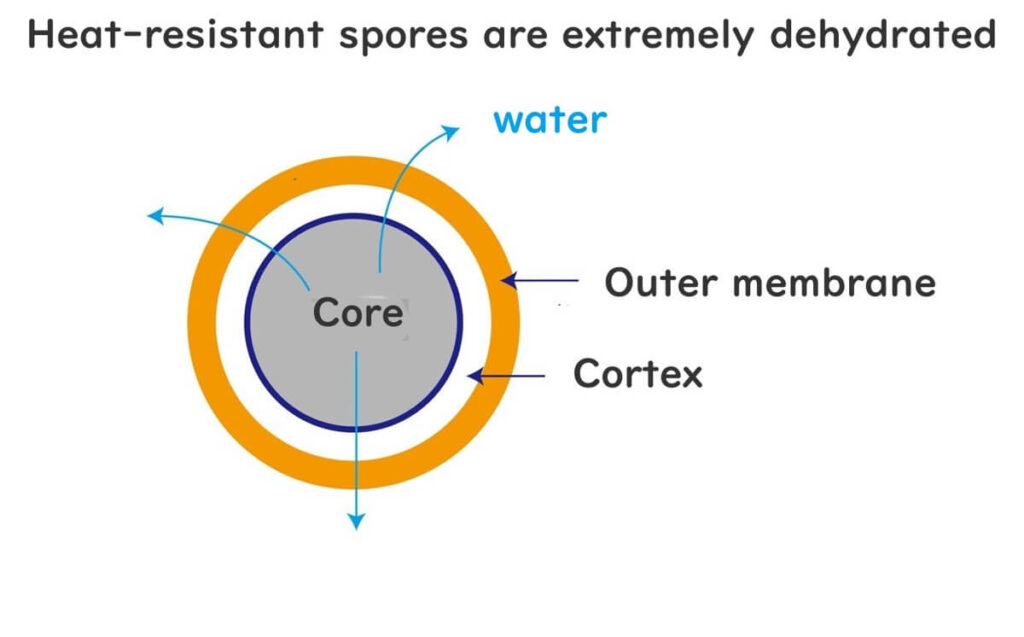
Heat-Resistant Spores Are Highly Dehydrated, Hence Their Resistance
Heat-resistant spores exhibit an extraordinary ability to survive even at high temperatures. While the detailed mechanisms behind this resilience are still not fully understood, one key factor has been clearly identified: these spores contain very little water.
During the process of spore formation, water is systematically removed from the cells, resulting in an extremely dehydrated state. This lack of water plays a critical role in the spores' heat resistance. Water is essential for heat to transfer efficiently and for protein denaturation, a process crucial for microbial sterilisation. In the absence of sufficient water, the heat's ability to damage microbial structures, such as proteins and DNA, is significantly reduced.
Thus, the dehydration of spores not only makes them resistant to high temperatures but also explains why traditional heat sterilisation methods, particularly dry heat sterilisation, struggle to effectively eliminate them. Understanding this principle helps guide the development of more targeted sterilisation strategies to combat these highly resilient microbes.