Let's chat about benzalkonium chloride, a disinfectant often found lurking in the corridors of food factories, standing shoulder to shoulder with sodium hypochlorite as one of the most commonly employed germ-slayers. Benzalkonium chloride is a household name in the world of disinfectants.
In our everyday lives, it's closer than you might think, popping up in plasters and even in some hand soaps. The secret behind its microbial massacre? It's all about the structure – sporting a hydrophobic functional group that's positively charged, forming a complex with negatively charged chloride ions.
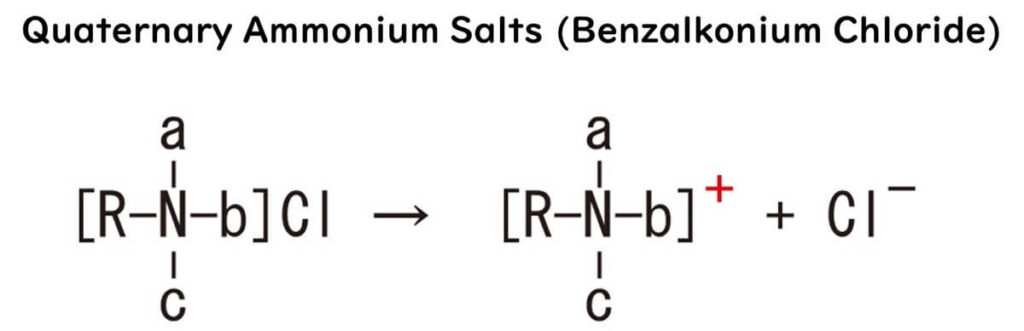
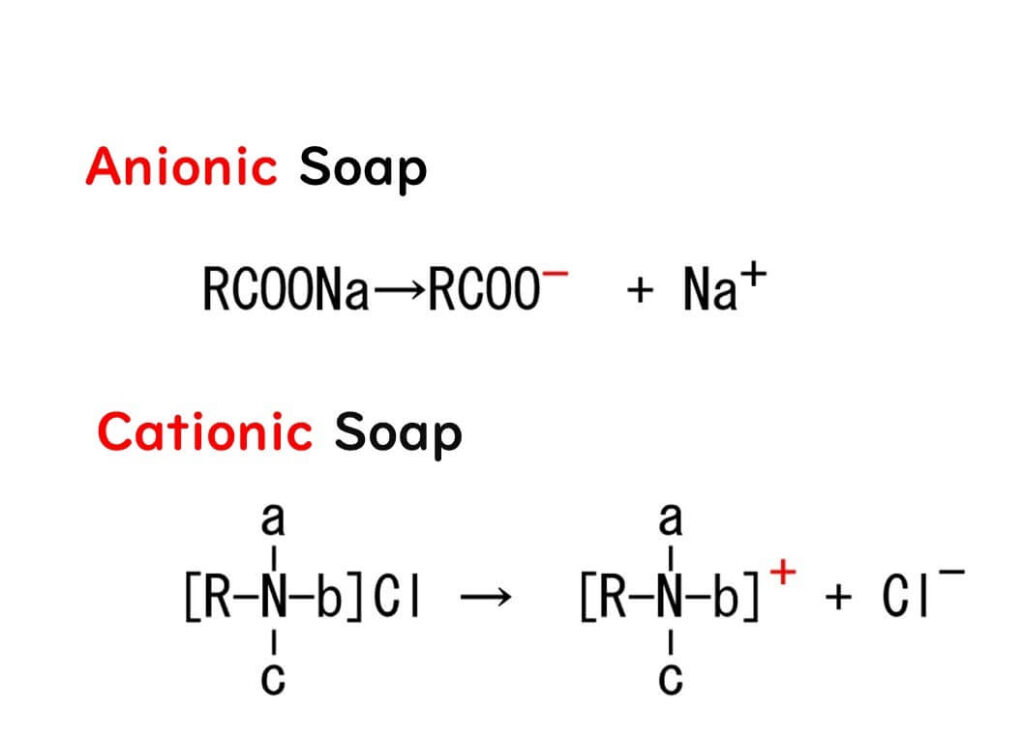
This gives it a sort of Jekyll-and-Hyde duality, hydrophobic on one side and electrically charged on the other, much like soap. Therefore, benzalkonium chloride can be considered a type of soap. However, unlike the most widespread soap made from sodium fatty acids, which is negatively charged, benzalkonium chloride swings the other way, being positively charged. So, while the former gets dubbed anionic soap, our friend benzalkonium chloride earns the titles of cationic soap or, due to its reverse electrical charge, reverse soap.
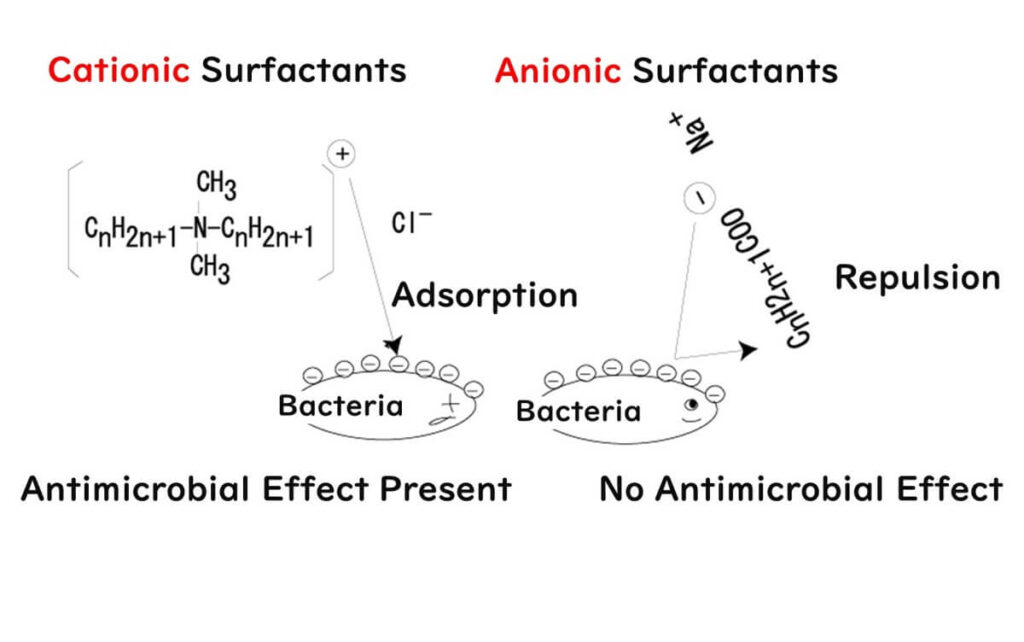
The killer move of benzalkonium chloride comes from its positively charged hydrophobic complex, a crucial detail when considering its bactericidal prowess. Why? Because microbial cell surfaces are negatively charged, making them irresistible targets for our positively charged hydrophobic complex – think of it as a guided missile homing in on its target.
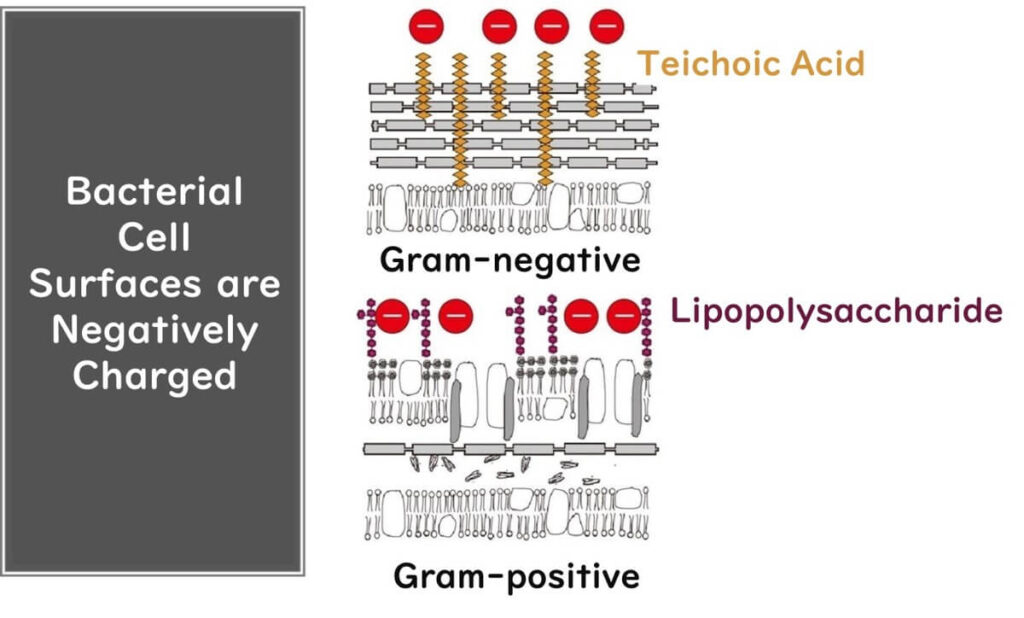
A bit on why microbes are negatively charged: for gram-negative bacteria, it's the outer membrane's polysaccharides that carry the negative charge. While Gram-positive bacteria lack this outer membrane, they do have teichoic acid sticking out from the cell wall – a polymer of glycerol phosphate that's also negatively charged. For our budding scientists reading this, the takeaway is that bacterial cell surfaces are negatively charged, a tidbit that's useful far beyond understanding the workings of benzalkonium chloride.
Speaking of workings, let's dive back into benzalkonium chloride. With its hydrophobic alkyl group bound to a nitrogen atom and the whole shebang carrying a positive charge, it forms a compound with negatively charged chloride ions. This setup allows it to pierce microbial cell surfaces like a missile, then, due to its affinity for the microbial cell membrane's hydrophobic parts, disrupt and puncture the membrane.
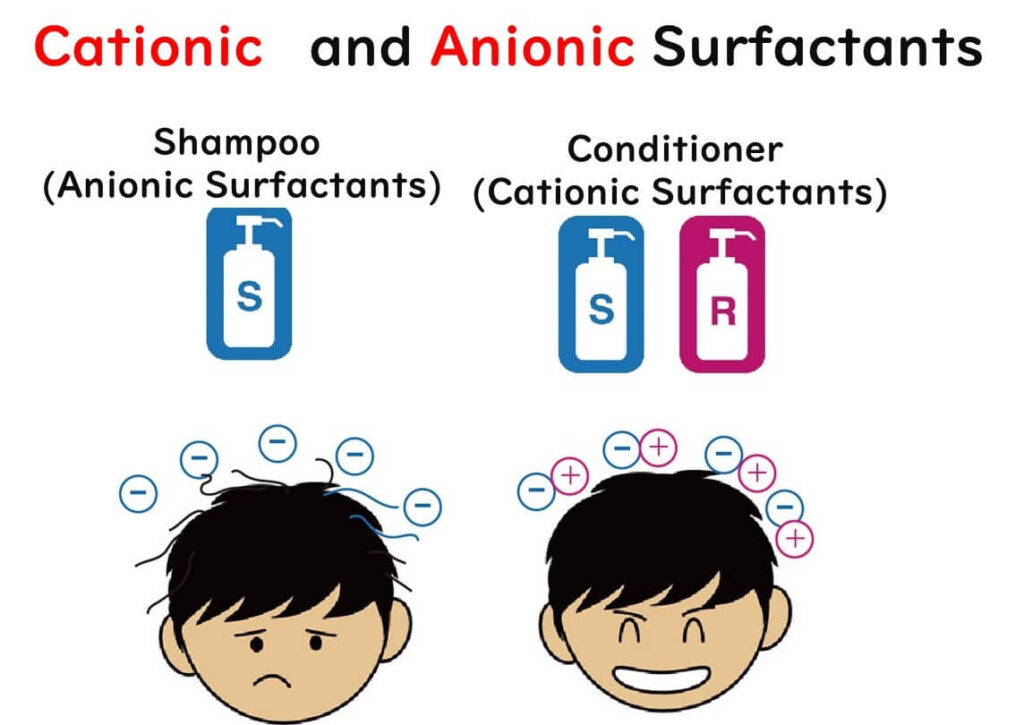
A word of caution for those in the food factory biz: don't mix anionic and cationic soaps.

Why? Because mixing negative and positive charges neutralizes them, rendering the bactericidal action of benzalkonium chloride ineffective. It's akin to the shampoo-and-conditioner scenario: shampoo leaves hair negatively charged and frizzy, while conditioner, being positively charged, neutralizes this, leaving hair smooth. Great for hair, not so much for killing bacteria.
Lastly, a note on drug-resistant bacteria. You might think that with disinfectants like alcohol, sodium hypochlorite, and benzalkonium chloride indiscriminately wrecking organic matter, resistance wouldn't be an issue. Generally, that's true, except for antibiotics, which target specific vulnerabilities.
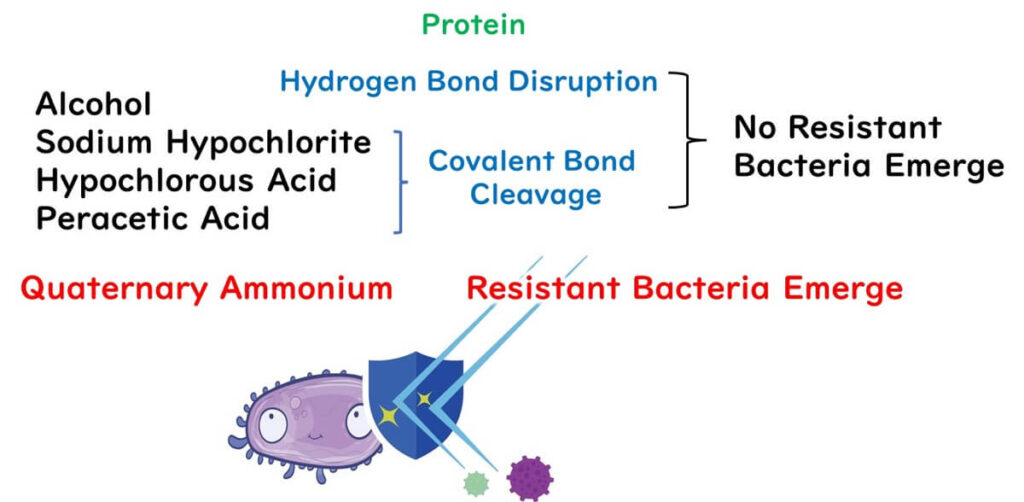
Surprisingly, though, there's been an uptick in research suggesting that benzalkonium chloride might not be immune to resistance after all, particularly with overuse in everyday settings. Something to keep in mind, especially for those of us frequenting the food factories.