In this article, we delve into the mysteries of Cronobacter sakazakii, a bacterium that raises concerns due to its ability to contaminate infant formula. We'll explore why this germ is particularly dangerous for infants, especially those who are vulnerable, and discuss essential safety practices when preparing baby formula to ensure the highest protection for little ones.
Where Does It Belong?
Cronobacter sakazakii is part of the big family of bacteria known as Enterobacteriaceae. It's a Gram-negative, facultatively anaerobic bacterium. Until 2007, it was grouped under the Enterobacter genus, but then it got its own 'Cronobacter' label.
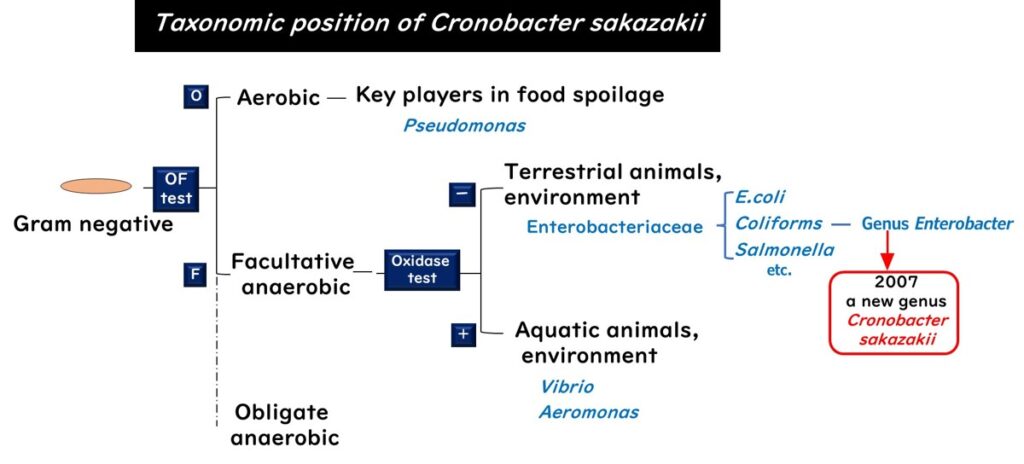
The name 'Cronobacter' is a bit of a dark nod to the Greek myth of Kronos, who had a bad habit of eating his children, referencing the bacterium's harmful effect on infants.

Where Can Cronobacter sakazakii Be Found?
This sneaky bacterium isn’t just confined to animal guts; it’s widespread in the environment, making it difficult to monitor consistently. Cronobacter sakazakii can appear in various unexpected places.
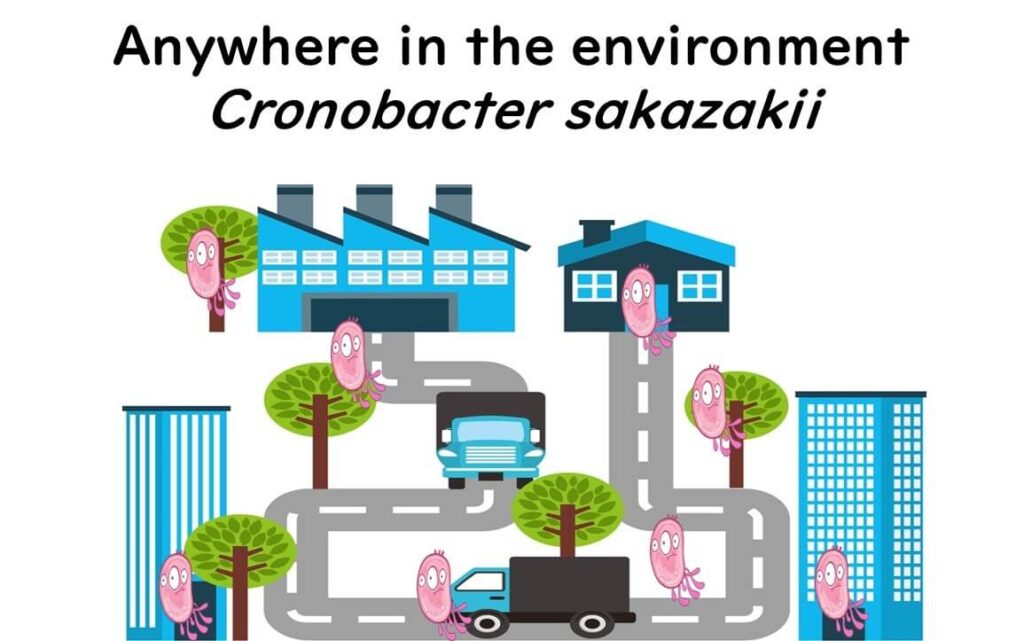
Foods commonly associated with Cronobacter sakazakii include herbal teas, spices, starchy foods, powdered soups, dried foods, and even salads. It has also been detected in food production facilities, especially where powdered infant formula is processed. Although it typically poses little risk to healthy adults, Cronobacter sakazakii presents a serious threat to infants, particularly in powdered formula.
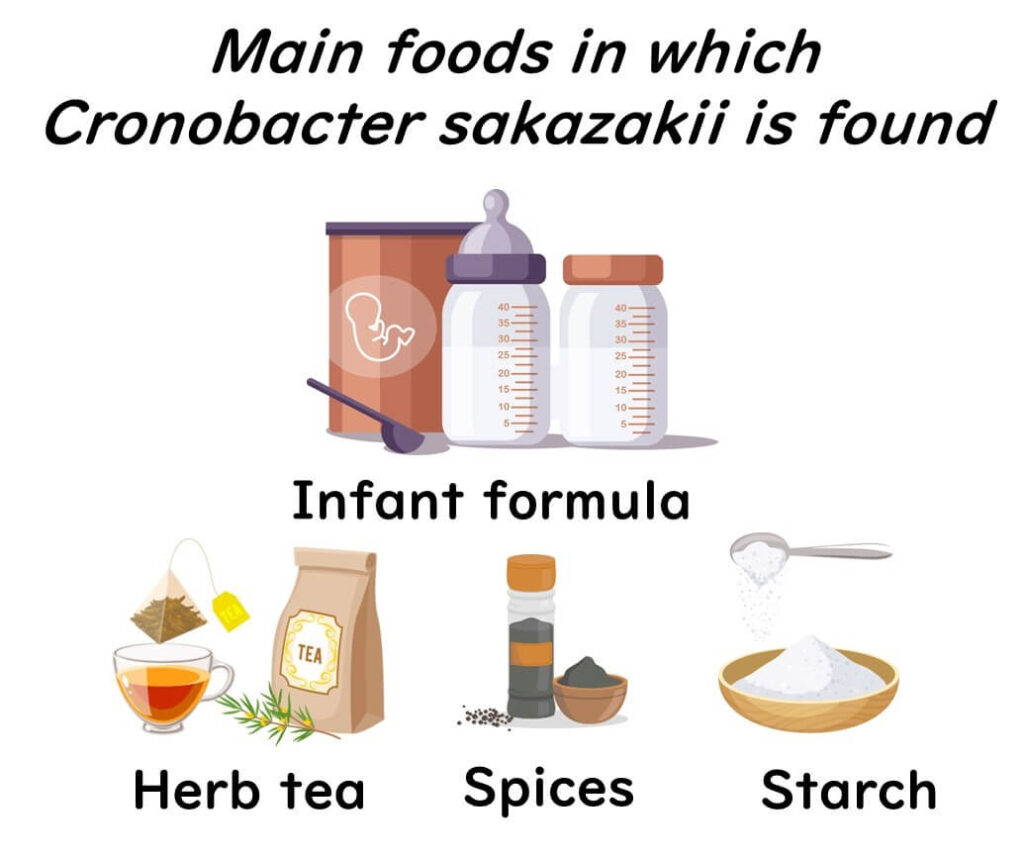
The Trouble Cronobacter sakazakii Causes
Cronobacter sakazakii is an equal-opportunity pathogen – while it can infect anyone, it poses a particularly severe risk to infants and elderly individuals with weakened immune systems. In adults, C. sakazakii infections are usually linked to postoperative infections or urinary tract issues.
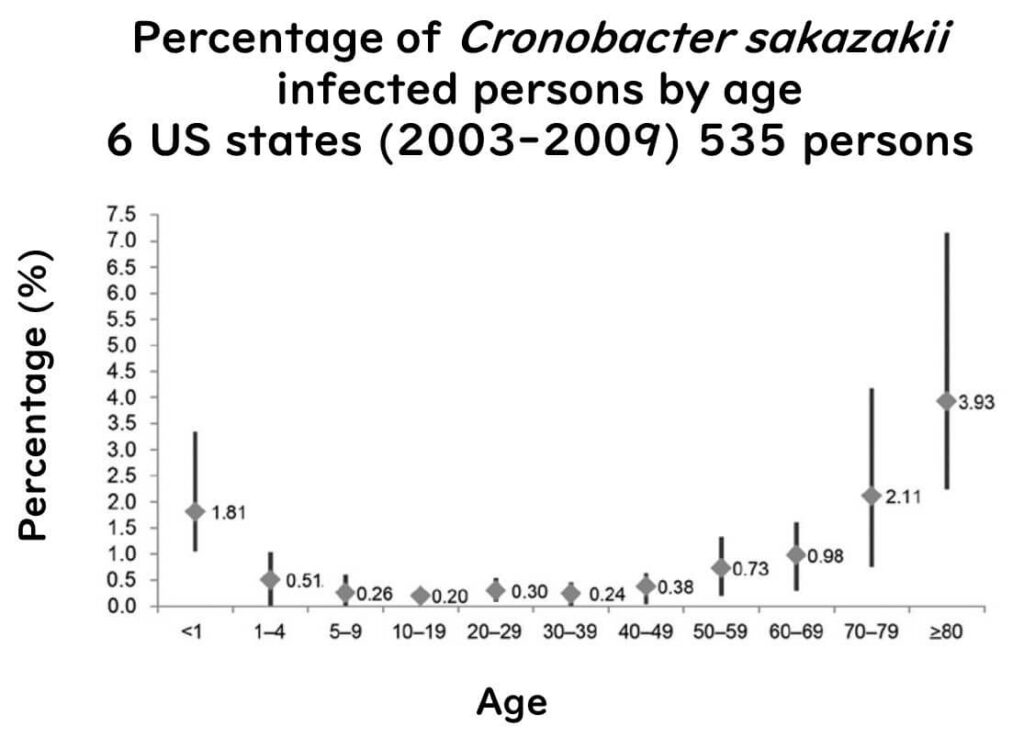
(Figure sourced from Emerging Infectious Diseases, Vol. 20, No. 9, September 2014, published by the CDC, in accordance with their public domain policy.)
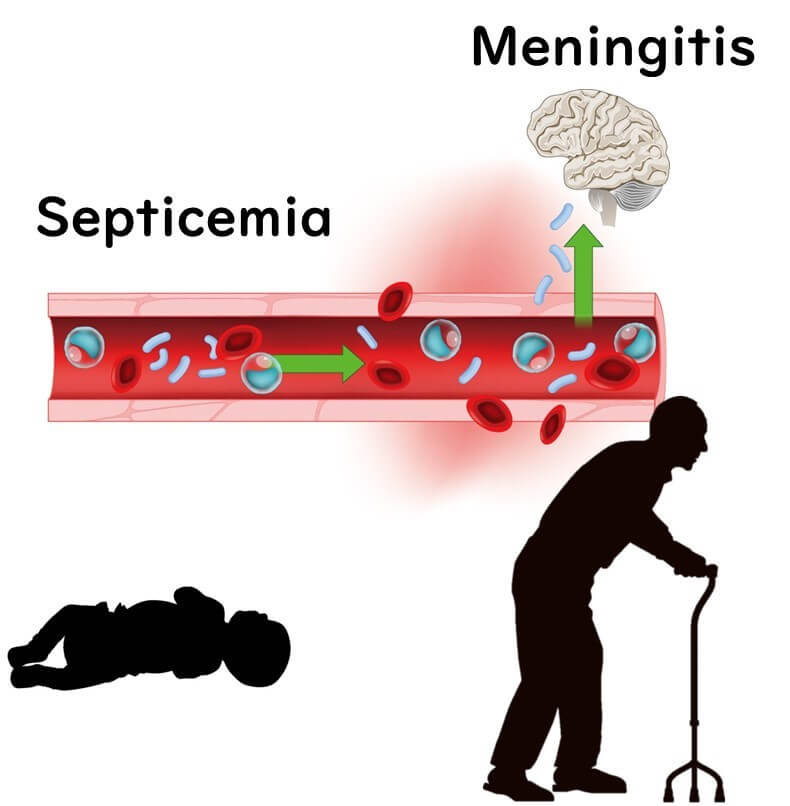
What makes this bacterium especially dangerous is its potential to cause serious, life-threatening conditions in newborns, especially those born prematurely or underweight. These infections can lead to severe illnesses such as meningitis, necrotizing enterocolitis (a damaging intestinal condition), and sepsis, with a sadly high risk of fatality. Even among survivors, complications like developmental delays can persist.

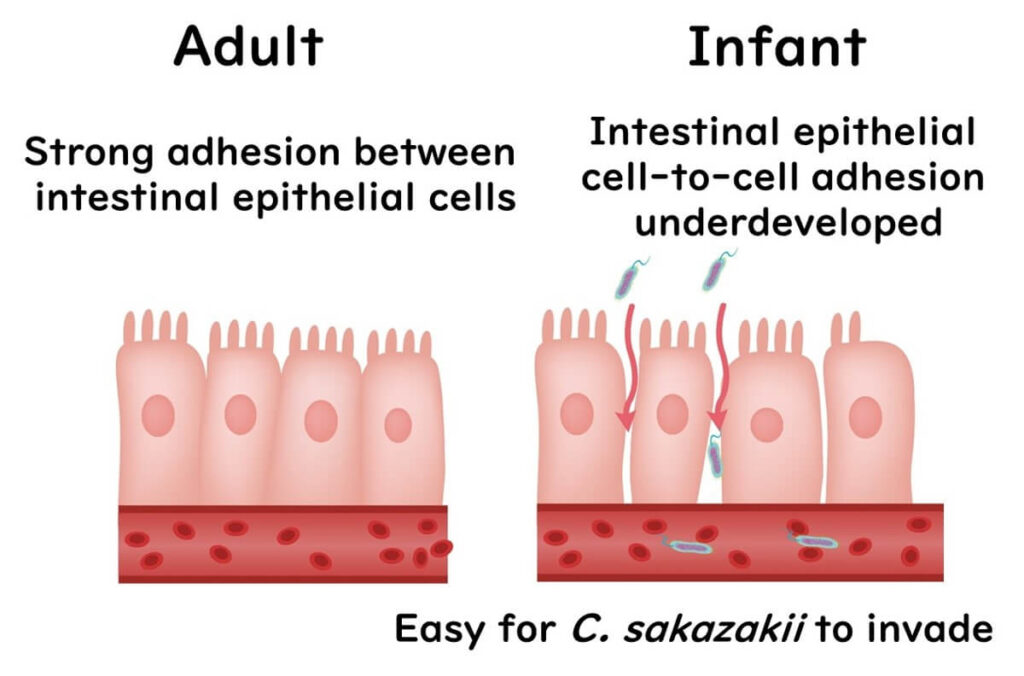
The bacterium appears to be adept at adhering to specific cells in the infant gut and may utilize particular proteins to cause further harm. Babies are particularly vulnerable as their intestines are not fully developed, making it easier for C. sakazakii to invade areas it shouldn’t. The primary conditions it causes in infants are sepsis and meningitis.
In the United States, C. sakazakii infections may be underreported. Currently, only Minnesota requires mandatory reporting when infections occur in infants under one year old, while other states do not have this requirement. Consequently, the CDC suspects that the true frequency of C. sakazakii infections in the U.S. may be higher than the official figures indicate.
The Notorious MLST ST4 in Cronobacter sakazakii
In the complex world of microbiology, identifying high-risk bacterial strains isn’t always straightforward. One effective method is MLST (Multi-Locus Sequence Typing), a genetic typing approach that classifies pathogens beyond their species. For Cronobacter sakazakii, 66 unique sequence types have been identified (source: www.pubMLST.org/cronobacter). In a study analyzing 41 clinical isolates of C. sakazakii, half (20 out of 41) were identified as sequence type (ST) 4. Notably, 9 out of 12 strains linked to cases of meningitis were found to be ST4. This highlights that ST4 is an especially virulent type of C. sakazakii, with a significant association with neonatal meningitis.
(Look out for an upcoming blog post where we’ll break down MLST technology in easy-to-understand terms!)
Additionally, certain strains of Cronobacter sakazakii have developed multidrug resistance, creating serious concerns about infections that are challenging to treat with conventional antibiotics.
Cronobacter sakazakii's Strength in Dryness
One reason Cronobacter sakazakii is such a concern in infant formula and other dried foods is due to its resilience under dry conditions. Although it's a Gram-negative bacterium from the Enterobacteriaceae family, and lacks the extreme dry-resistance abilities of Gram-positive bacteria, C. sakazakii is still recognized as one of the toughest Gram-negative bacteria in low-moisture environments.

A study examining the survival of various Enterobacteriaceae under dry conditions categorized them into three groups based on their resistance to dryness:
- Low Resistance Group: This includes Citrobacter koseri, Citrobacter freundii, and Enterobacter cloacae, which could not be detected on dry surfaces after six months.
- Intermediate Resistance Group: This group, including Salmonella Enteritidis, Escherichia coli, and Klebsiella pneumoniae, managed to survive up to 15 months in dry conditions.
- High Resistance Group: The most resilient group, consisting of Cronobacter sakazakii, Escherichia vulneris, Klebsiella oxytoca, and Pantoea spp., remained detectable even two years later.
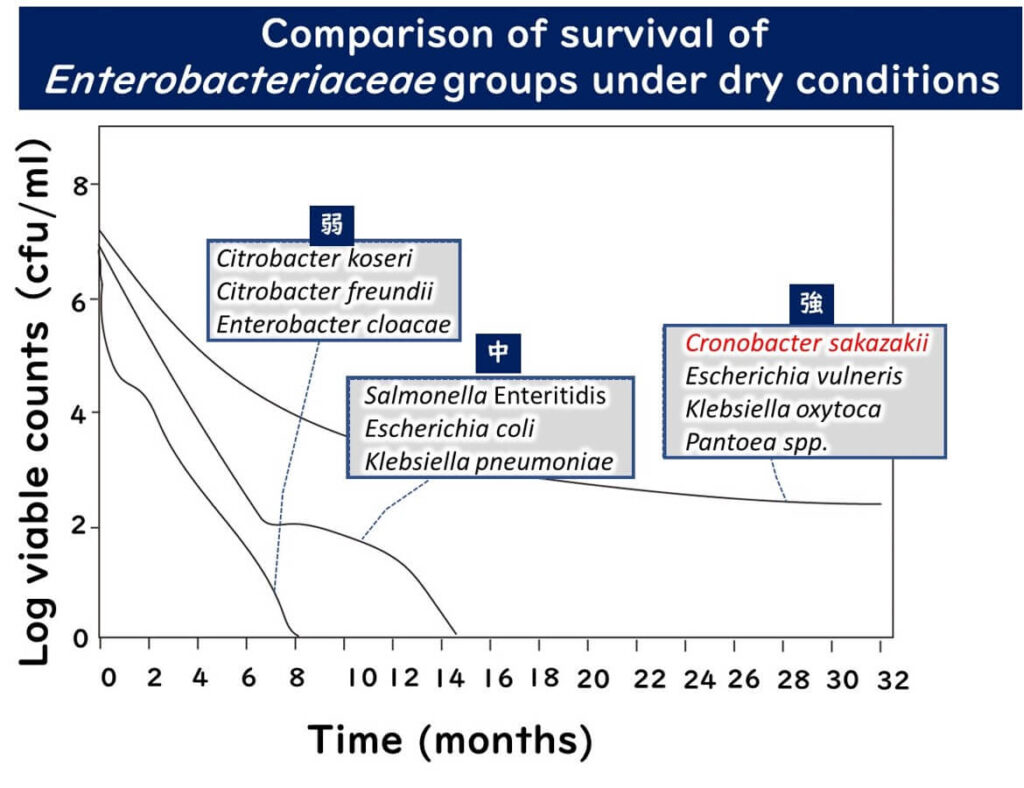
The figure above was recreated from data presented in the study,
Dry stress and survival time of Enterobacter sakazakii and other Enterobacteriaceae in dehydrated powdered infant formula
J Food Prot. 2007 Sep;70(9):2111-7.
Thus, Cronobacter sakazakii is categorized among the most dry-resistant Enterobacteriaceae, posing an ongoing contamination risk in powdered infant formula and other dried foods, potentially leading to severe infections.
The Role of Capsules in Cronobacter sakazakii's Resistance to Dryness
Why does Cronobacter sakazakii, a Gram-negative bacterium in the Enterobacteriaceae family, exhibit such resilience in dry conditions? What mechanisms contribute to this hardiness?
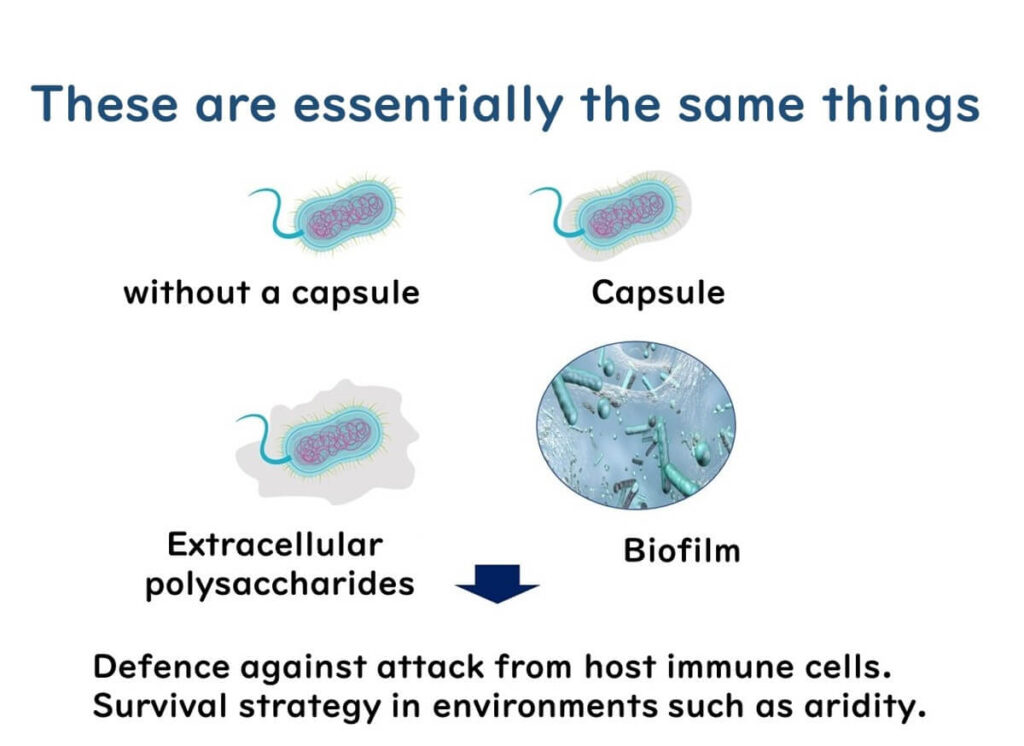
Not all strains of Cronobacter sakazakii form capsules or extracellular polysaccharides, but many do. To understand their role, it helps to clarify the structures involved:
- Capsule: The term 'capsule' might imply a rigid shell, but it’s actually a uniform layer of high-molecular-weight polysaccharides secreted by the bacteria to coat the cell. This structure shares similarities with the material found in biofilms.
- Extracellular Polysaccharides: These are polysaccharides secreted by the bacteria into their surrounding environment, though they are less structured than capsules.
- Biofilms: Biofilms form when bacteria secrete polysaccharides between cells, creating a collective, protective layer on solid surfaces.
Understanding that capsules, extracellular polysaccharides, and biofilms are essentially variations of the same material with structural differences can provide insights into bacterial survival strategies.
The production of these structures varies by bacterial strain and environmental conditions. In many pathogens, capsules serve as a defense against host immune cells, while biofilm formation can help bacteria survive in harsh environments. For instance, pathogens often form capsules when infecting animal hosts, though they might not produce them in pure culture conditions. When environmental conditions worsen, bacteria may also form biofilms on surfaces.
In the case of C. sakazakii, many strains have been observed to produce capsules and extracellular polysaccharides. Research comparing Enterobacteriaceae survival in dry conditions found that:
- During the first 18 months of dry storage, there was no clear link between capsule formation and recovery.
- After two years, 4 out of 5 strains that could still be recovered were capsule-forming.
- At 2.5 years, only two strains with capsules were recoverable.
These findings suggest that capsule formation may be a crucial factor in the long-term survival of Cronobacter sakazakii in dry environments, allowing it to persist in powdered foods such as infant formula.capsule plays a significant role in the long-term survival of Cronobacter sakazakii under dry conditions.
Contamination Patterns in Infant Formula
Preventing Cronobacter sakazakii contamination entirely in infant formula manufacturing is a global challenge (CDC). One key reason is that the essential nutrients in infant formula can be damaged by heat sterilization, leading manufacturers to add these nutrients after heating. As a result, there’s a risk of bacterial contamination in the final packaging stage, even under stringent controls.
Note: Manufacturing processes may vary by manufacturer. The information here outlines general practices on an international scale.
Additionally, since Cronobacter sakazakii is prevalent in everyday environments, contamination risks extend beyond manufacturing. For instance, when parents or caregivers prepare powdered infant formula with hot water at home, there is still a possibility of exposure to this bacterium.

Preparing with Water at 70°C
The WHO provides specific guidelines for preparing powdered infant formula at home, advising that water hotter than 70°C should be used to dissolve the powder. But why exactly 70°C?
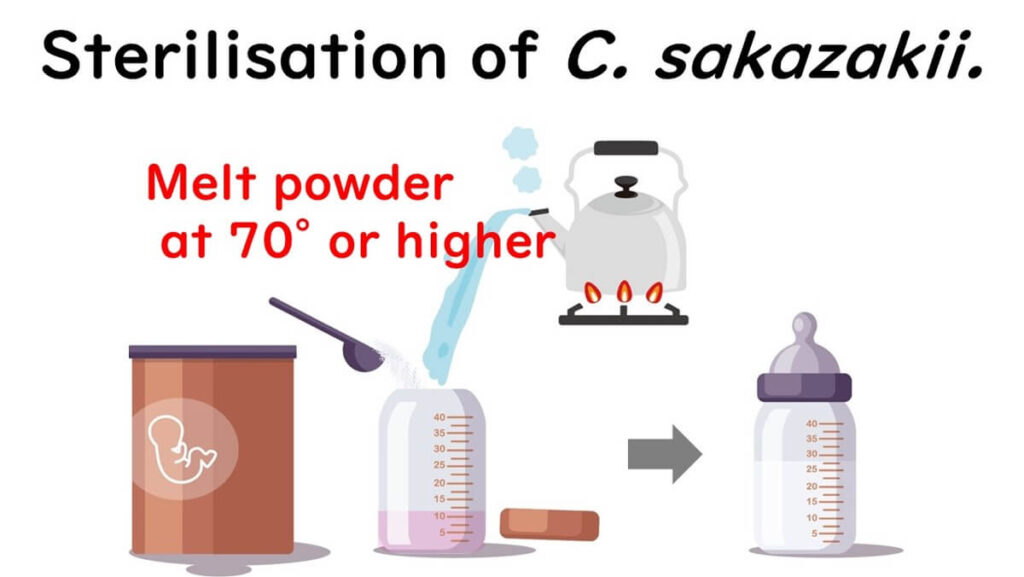
This recommendation ties directly to the concept of the D-value, a critical measure in microbial sterilization. The D-value indicates the time required to kill 90% of bacteria at a given temperature. For example, a D-value of 2 minutes at 70°C means that 90% of bacteria would be destroyed in 2 minutes, allowing for safe food without compromising its quality.
Let's break down the calculations:
Research on Cronobacter sakazakii found a D-value of 3.9 seconds at 70°C. Given that contamination levels of Cronobacter sakazakii in powdered milk are generally low, at 1 cfu/100g or less, applying a 4D heat treatment at 70°C—3.9 seconds × 4 = 15.6 seconds—can reduce bacterial presence to an effective 0.0001 cfu/100g. In practical terms, this calculation suggests that the risk of infection by Cronobacter sakazakii can be virtually eliminated by heating powdered milk at 70°C for the recommended time.
Understanding and applying this D-value concept allows parents and caregivers to effectively prevent Cronobacter sakazakii infections, ensuring safer formula preparation for infants.

Liquid Milk is the Safest Option
Unlike powdered infant formula, liquid infant milk sold in bottles or cartons undergoes sterilization either after packaging or as part of the liquid sterilization process before packaging in a sterile environment. This significantly lowers the risk of Cronobacter sakazakii contamination, making liquid milk a safer choice. The CDC especially recommends liquid milk for infants under three months whenever possible, as it virtually eliminates the contamination risk associated with powdered formulas.
In Japan, following updates to the 'Ministerial Ordinance on Component Standards of Milk and Dairy Products' and the 'Standards for Foods, Additives, etc.' by the Ministry of Health, Labour, and Welfare in 2018, the production and sale of liquid infant milk have been made possible domestically. This change supports a safer feeding option for infants in Japan, aligning with international best practices for infant safety.