In this article, we'll dive into the intriguing world of water activity, cleverly deciphering its principles and how it differs from mere water content. We'll explore the nuances of bound and free water, the principles behind measuring water activity in food, and how reducing water activity can enhance the shelf life of preserved foods like those cured with salt. Additionally, we'll discuss the relationship between water activity and the growth of microbes such as bacteria and mold. Plus, I'll toss in some handy values of water activity to remember that could be a lifesaver—or at least a food saver! So, buckle up, and let's get our science on!
What is Water Activity?
So, what exactly is water activity? In a nutshell, it refers to the degree of water available for microbes to use, expressed as a numeric value. Take dried shiitake mushrooms, for example—they inherently lack sufficient water by absolute amount. In the case of pickles and jams, they're brimming with dissolved salt and sugar respectively, which hog all the solvent—water, in this case—leaving less available water (free water) for microbial use.
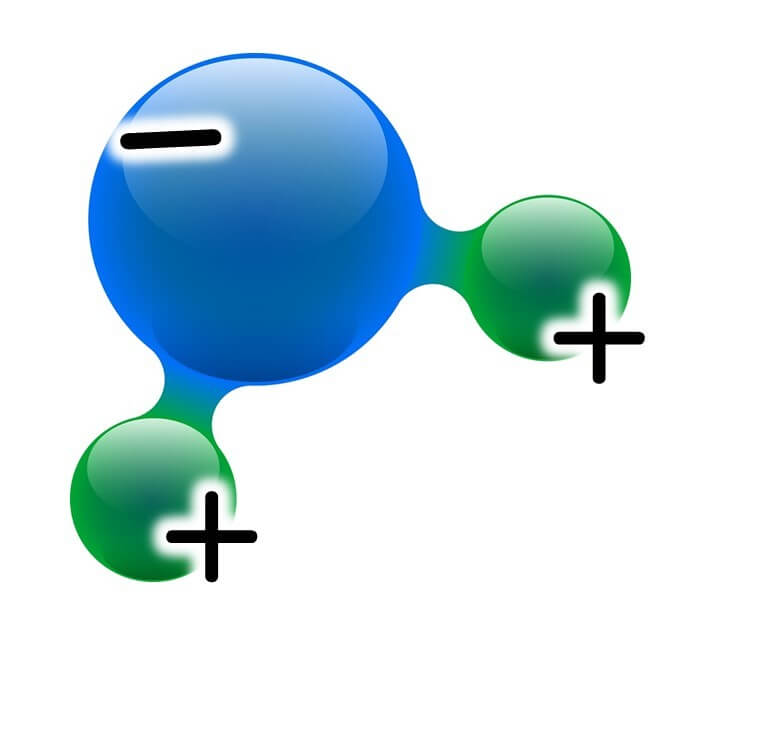
For instance, in saltwater, salt dissolves into sodium and chloride ions, around which water molecules cluster and get trapped.
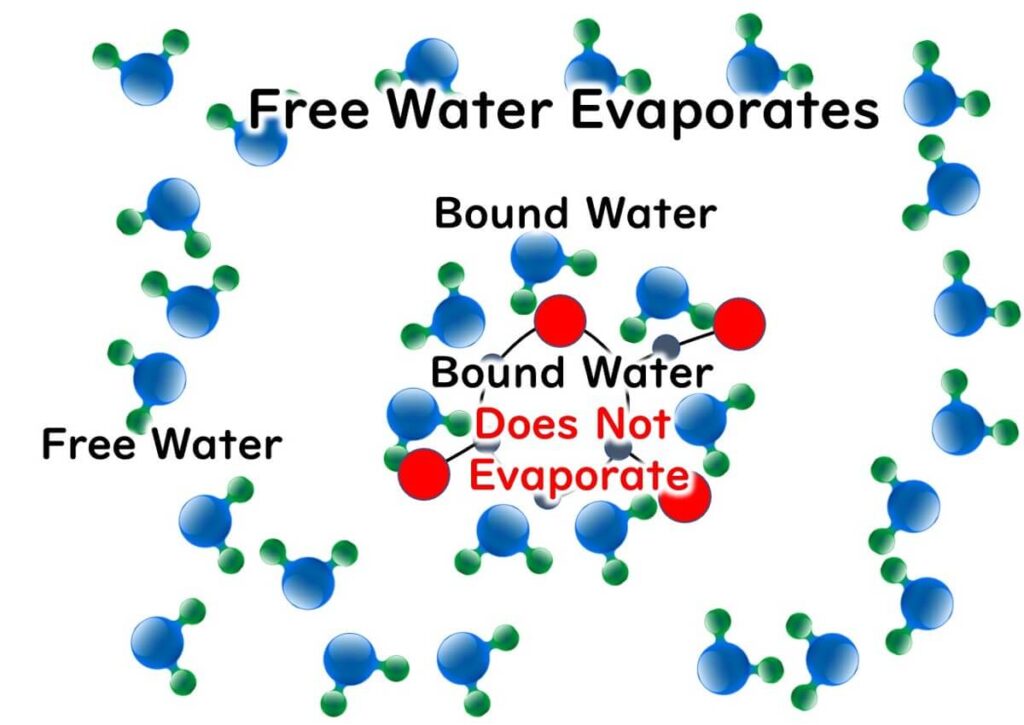
This means there are fewer water molecules (free water) that can move freely compared to in unsalted water.
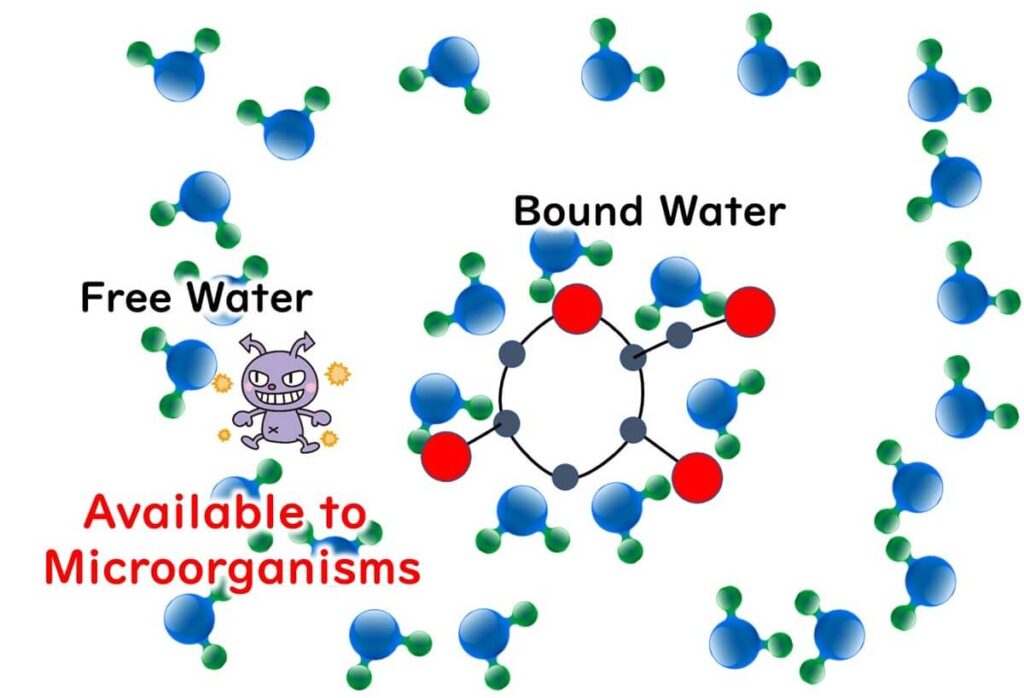
This concept of osmotic pressure, crucial for the proliferation of microbes, is expressed in the food sector as water activity (Aw). It's applied in preservation methods such as salting and sugaring.
Foods That Lower Water Activity
Why is water activity a handy metric in controlling foodborne microbes? Let's consider this scenario: imagine we have some bread and strawberry jam. If you only consider the amount of water, the bread appears drier. However, while mold grows on bread, it doesn't on the jam-packed with moisture. By comparing their water activities, bread has a water activity of 0.96, whereas strawberry jam has a lower water activity of 0.76. This lower water activity in the jam means it's less prone to microbial spoilage.
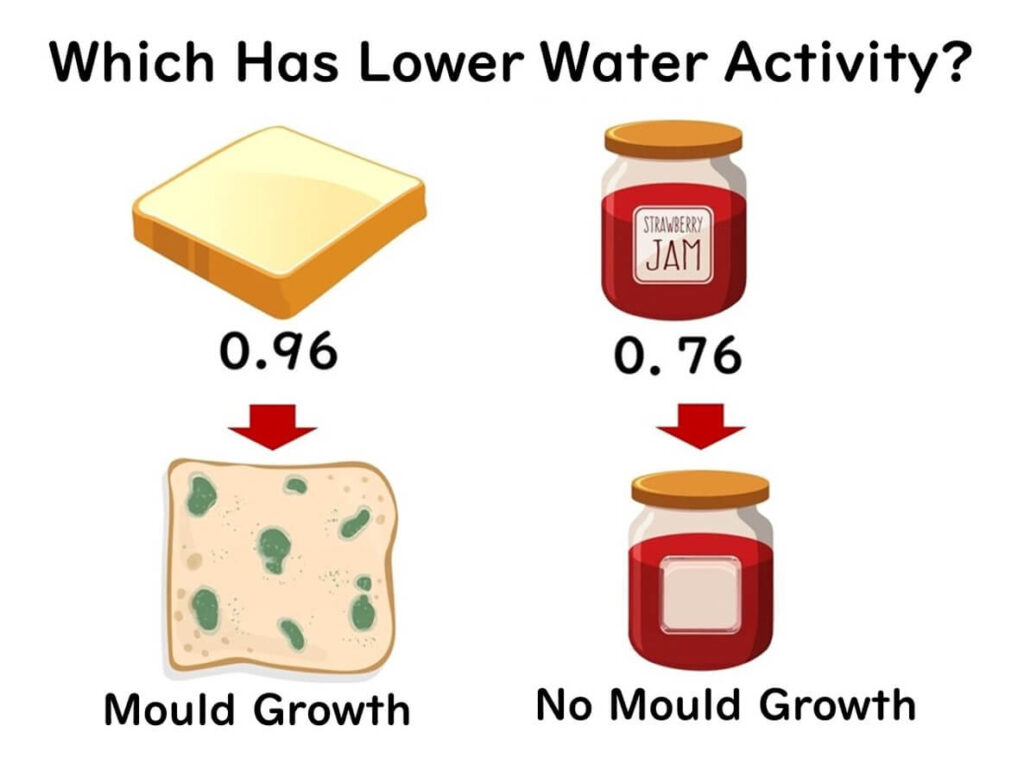
This shows how the concept of water activity can provide a more useful measure than just water content when assessing the likelihood of microbial growth.

Methods of Measuring Water Activity
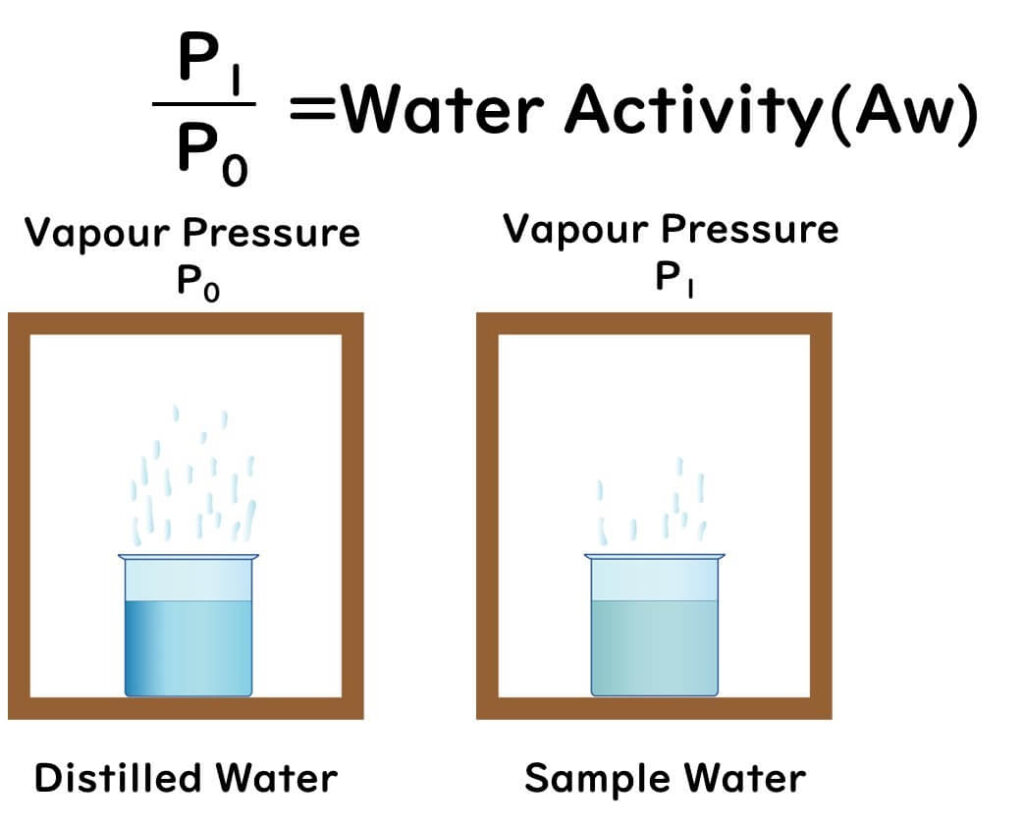
Water activity is defined as follows:
- It is the ratio of the vapor pressure (P) in a sealed container at a constant temperature, containing the food product, to the maximum vapor pressure (P0) at that temperature, expressed as P/P0 = Aw.
For example, a water activity of 0.9 means:
- "The saturated vapor pressure measured from a cup of water dissolved with salt or sugar inside a box" ÷ "The saturated vapor pressure measured from a cup of pure water inside a box (100% humidity)" = 0.9
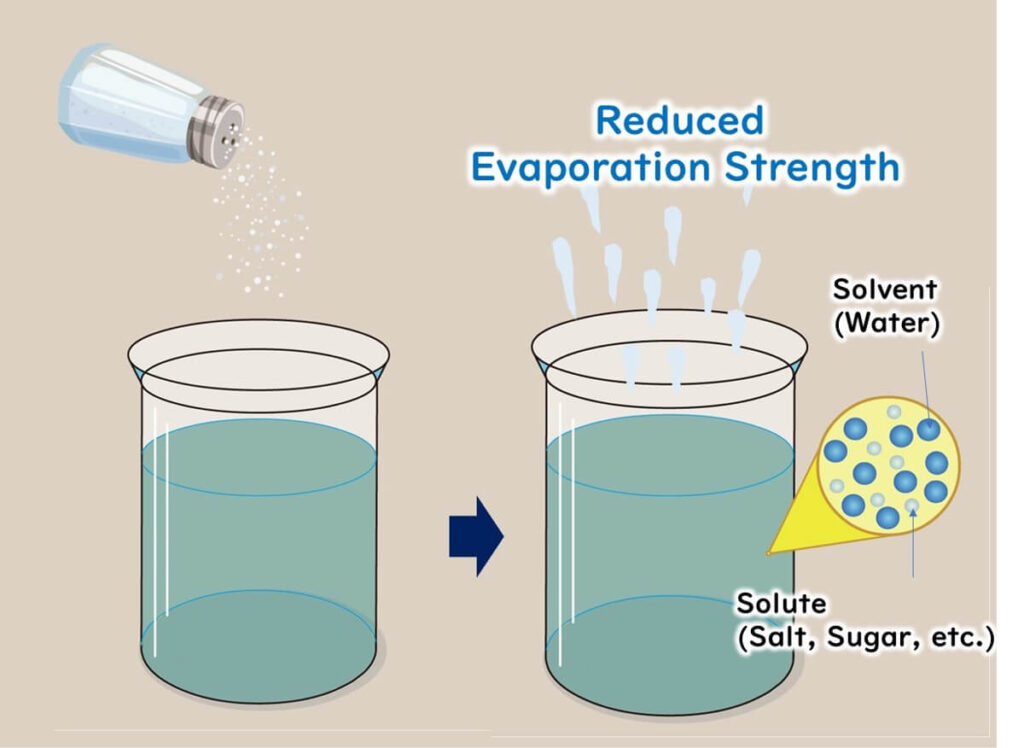
Measured over a certain period, the evaporation rate of water with dissolved salt or sugar is lower than that of pure water, resulting in a water activity value below 1.0 (pure water).
Water activity measurements are typically conducted at 25°C, with most water activity measurement devices capable of measuring within a range of 15-35°C.
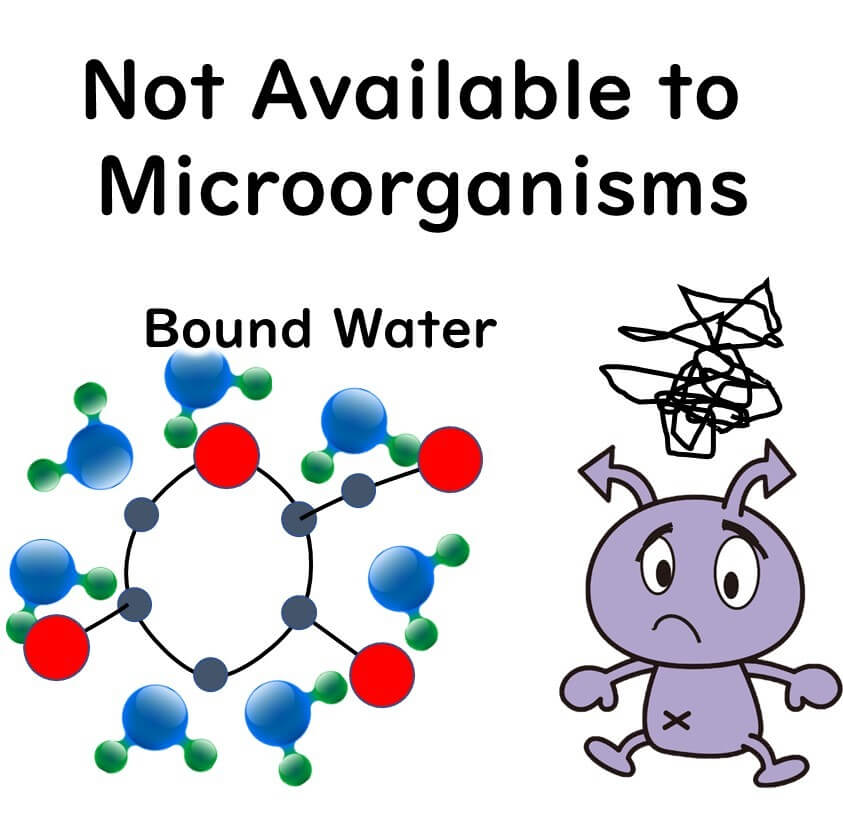
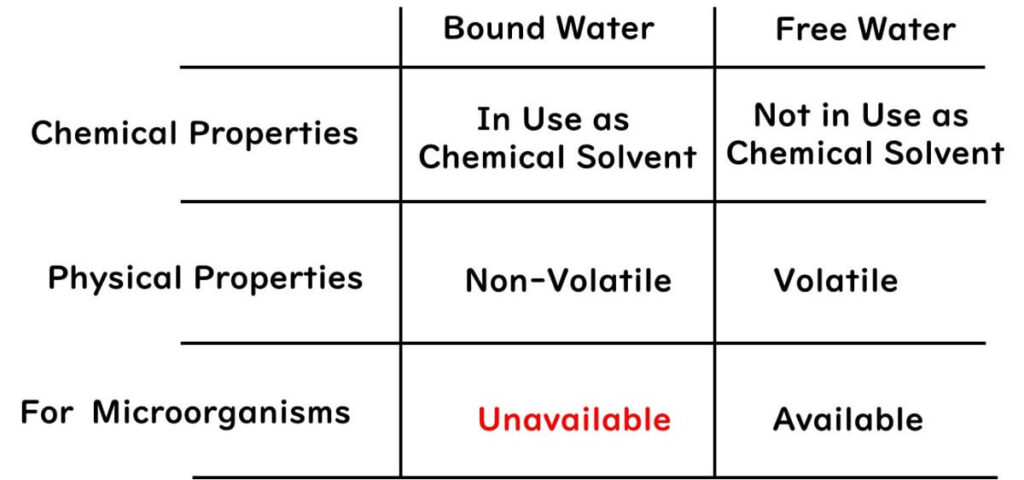
When it comes to bound and free water related to water activity, here's a brief overview:
1.Chemical Properties: Bound water serves as the solvent in chemical substances. Free water is water that does not act as a solvent.
2.Physical Properties: Bound water does not evaporate, whereas free water does.
3.Properties Concerning Microbes: Microbes cannot use bound water, but they can use free water.
Microbial Growth and Water Activity
In the microbial world, mold, yeast, and bacteria exhibit varying degrees of tolerance to water activity. For instance, dried mochi left out will eventually develop mold due to its low water activity. High-salt pickles and high-sugar jams are also prone to spoilage by molds and yeasts due to the same reason. The minimum water activity needed for microbial growth ranges from 0.90 for bacteria, 0.88 to 0.60 for yeast, and 0.80 to 0.60 for molds. However, understanding the concept of water activity and remembering the figures is one thing, practically applying this knowledge is another.
Let's consider a real-life story from the late 1990s. During a significant outbreak of E. coli O157 food poisoning in Japan, an alumnus of mine was concerned about potential contamination in the confectionery products his company sold. I asked him about the water activity of the confectionery. He couldn't immediately recall the figures, a clear sign that while the theoretical knowledge was there, the practical application was lacking. He returned the call the next day to report that the water activity was 0.8. I reassured him, "Then you don't need to worry about E. coli O157; you've just wasted two valuable workdays."

This anecdote underscores the importance of not only understanding water activity theoretically but also possessing practical knowledge of critical values. Two particularly useful figures to keep in mind are 0.85 and 0.7. These figures can help prevent microbial spoilage and ensure food safety more effectively than merely relying on theoretical knowledge.
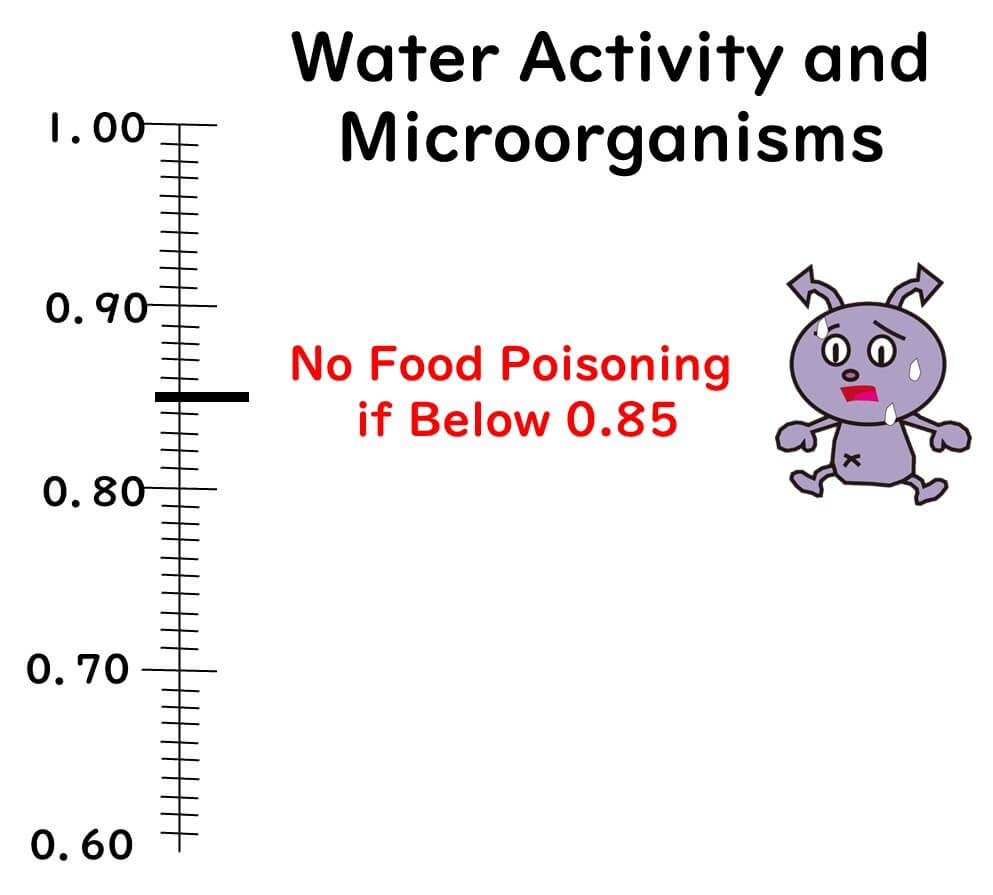
No Concern for Foodborne Bacteria Below Water Activity of 0.85
First up, let's discuss the significance of the 0.85 threshold.

Below this level, the growth of foodborne bacteria does not occur. Why is that? It's because the bacterium considered to be the most robust against low water activity, Staphylococcus aureus, has a minimum growth water activity of around 0.86. Thus, with a product that has a water activity of 0.85, you can essentially rule out the risk of bacterial food poisoning.
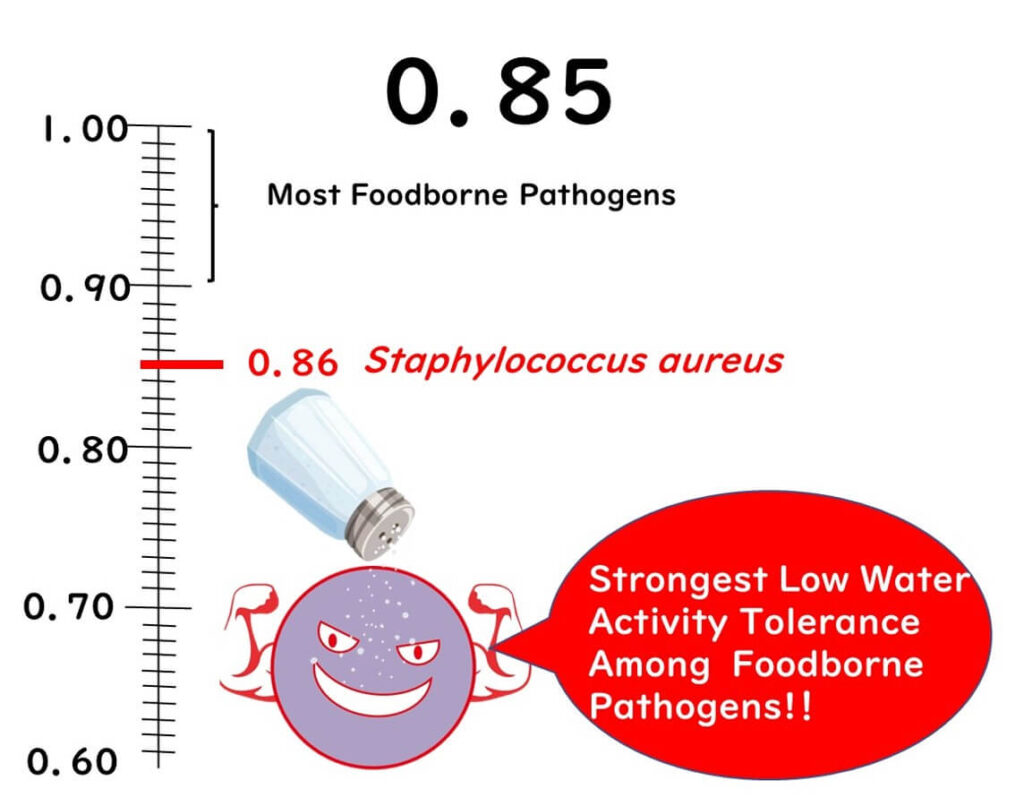
It's worth noting that Staphylococcus aureus is particularly resilient in low water activity environments. Most gram-negative bacteria, which include many pathogens, and other common gram-positive bacteria cannot grow at water activities below 0.9.
Keeping this in mind helps in understanding the broader context of microbial growth in relation to water activity.
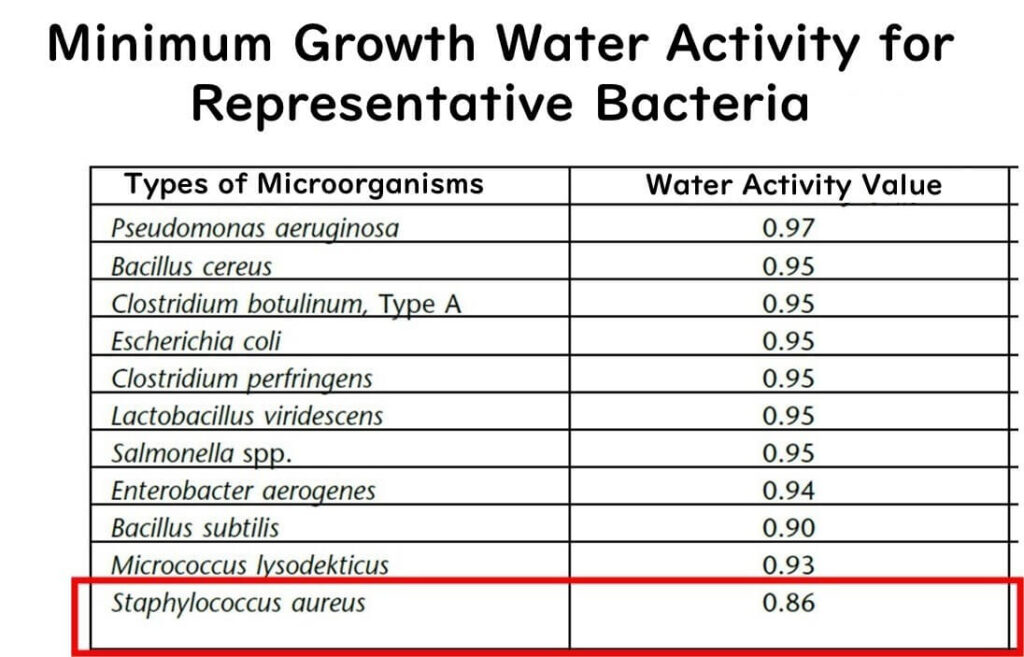
Extracted from United States Pharmacopoeia (USP) documents.
No Mold Growth Below Water Activity of 0.70
The next handy number to remember is 0.70. Products with a water activity below 0.70 are generally safe from mold growth. This is because the growth limit for most molds that cause problems in food is around 0.75.

Understanding water activity is not just about memorizing textbook figures but also about organizing practical values in your mind for effective use.

These numbers, 0.85 and 0.70, provide clear benchmarks to help ensure food safety and quality, reducing the risk of spoilage and foodborne illness.

Commonality in Salt Curing, Drying, and Freezing: Deprivation of Water Available to Microbes
Understanding water activity illuminates a common principle in traditional food preservation methods. Whether it's pickles through salt curing, jams via sugar curing, or dried products like dried fish, the key element they all share is the removal of free water from the food. This reduction in water activity is a fundamental concept that unites these various methods of preservation.
nterestingly, freezing can also be conceptualized similarly in terms of microbial growth inhibition due to reduced water activity. When food is frozen, the water within it becomes unavailable to microbes, much like in other methods of reducing water activity.
Note: However, it's important to clarify that water activity, as discussed, typically applies within a non-frozen range (generally 15-35°C) in the food industry. There's no practical need, nor are there common commercial devices, to measure the water activity of frozen foods because, theoretically, if measured, the water activity value of frozen food would approach 1.0 due to the freezing of the pure water itself, which lowers its vapor pressure. Hence, directly applying water activity to frozen foods doesn't fit neatly. But for the sake of understanding, here we discuss freezing in the context of how it sequesters free water from microbes, aligning it with other preservation methods.
※For a deeper dive into how freezing affects microbial growth and lethality, see the article
"Freezing and Microbial Mortality."
In summary, the reason dried shiitake mushrooms don’t spoil, pickles don’t rot, and jams don’t spoil—or why frozen foods remain preserved—is microbiologically the same: it’s all about depriving microbes of free water, a principle understood through the concept of water activity.
Note on Freezing: Unlike curing and drying, freezing doesn't just remove free water; it also introduces the element of 'low temperature,' which contributes additionally to microbial control.
Controlling microbial growth through temperature control