In this article, we’ll delve into a unique question in microbiology: Why don’t lactic acid bacteria have heme, and how does this relate to their catalase-negative nature? Understanding this distinct trait sheds light on the metabolic adaptations that enable these bacteria to thrive in various environments without relying on heme-dependent enzymes like catalase.
The Role of Heme in Bacterial Metabolism
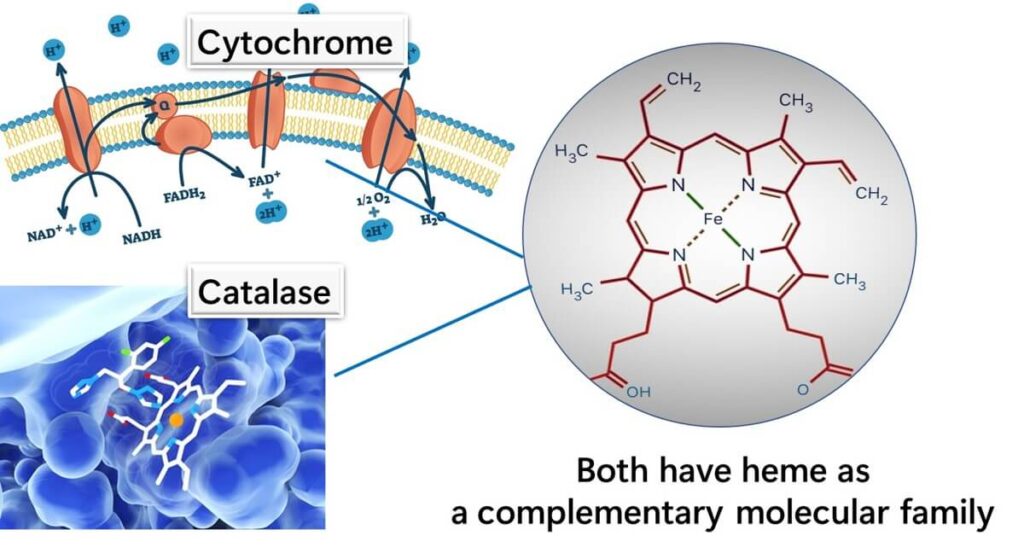
Most bacteria utilize the electron transport chain as part of aerobic respiration, and within this system lies the crucial component, cytochrome, which contains a heme structure. This heme-based cytochrome is essential for electron transport and energy production, supporting cellular growth in an oxygen-rich environment. For bacteria that engage in aerobic respiration, heme is therefore vital.
Lactic Acid Bacteria’s Unique Metabolism
Unlike many other bacteria, lactic acid bacteria exclusively rely on lactic acid fermentation for their energy needs, bypassing the electron transport chain altogether. Because they do not use this chain, they lack cytochrome and therefore do not require heme. Their growth and survival depend entirely on fermentation, making them unique among Gram-positive bacteria.
Why Lactic Acid Bacteria Lack Catalase
Since catalase also depends on heme to function, the absence of heme in lactic acid bacteria means they cannot produce catalase. Catalase typically breaks down harmful hydrogen peroxide into water and oxygen. Instead of catalase, lactic acid bacteria use an alternative enzyme called NADH peroxidase, which incorporates FAD (flavin adenine dinucleotide) rather than heme, to neutralize hydrogen peroxide. This adaptation allows lactic acid bacteria to survive without a heme-dependent mechanism.
Conclusion
In summary, lactic acid bacteria’s lack of heme is due to their reliance on lactic acid fermentation over aerobic respiration. This metabolic choice means they have no need for the electron transport chain or cytochrome, thus eliminating the need for heme. Consequently, they cannot produce catalase but have evolved an alternative mechanism with NADH peroxidase to handle oxidative stress, underscoring their unique place in the microbial world.